BACKGROUNDNumerous papers over eight decades since 1935 have reported that intestinal glucose absorption comprises two components, one secondary active and mediated by SGLT1, the other a highly regulated “diffusive” component occurring predominantly at concentrations well above those required to saturate SGLT1. In 1987, Pappenheimer proposed that the “diffusive” component was mediated by SGLT1-dependent solvent-induced paracellular flow through tight junctions, as a result of the concentration of glucose up to ~300 mM in the intercellular spaces
2. The field was split by the ensuing debate in which Diamond and Ferraris contested the concept of paracellular flow
3-6. In so doing, they implied that SGLT1 is the only pathway of glucose absorption and denied the existence of two components.
Subsequently, Kellett’s (my) laboratory proposed that the “diffusive” component is mediated by the glucose- and SGLT1-dependent transient insertion of the low affinity basolateral transporter GLUT2 into the apical membrane at high glucose concentrations, so that GLUT2 then mediated a major pathway of transcellular glucose absorption
7-9. Detailed mechanisms for the regulation of apical GLUT2 insertion by diet, sugars, peptides, Ca
2+, artificial sweeteners, hormones and obesity have been described
10-18. In effect, the apical GLUT2 model replaced paracellular flow and the impasse in the long-standing debate seemed resolved.
Nevertheless, as cited, some workers have recently reported difficulty detecting apical GLUT2. They therefore continued to deny the existence of any significant pathway other than SGLT1. In fact, the known properties of apical GLUT2 dietary regulation explain both cases. In the report by Gorboulev
et al.19,20 mice were starved, which reduces apical GLUT2 and enhances SGLT1; mice also have higher SGLT1 activities than rat. In the report by Roder
et al. 21 using SGLT1 knockout mice, KO and wild-type animals were necessarily maintained on sugar-free diets, which are well known to diminish apical GLUT2 greatly.
17 A NOVEL PARACELLULAR FLOW PROPOSALProfessor Naftalin has undertaken the truly daunting task of seeking to provide a comprehensive, integrated model of intestinal glucose absorption. He accepts there are two components of glucose absorption and also that GLUT2 is transiently inserted into the apical membrane. However, in a further twist to the debate, he seeks to reinstate a central role for paracellular flow and thereby reinterpret that of apical GLUT2.
In essence, it is proposed that glucose transport through SGLT1 results in accumulation of cytosolic glucose to supraluminal concentrations in enterocytes. At that point, net absorption of glucose through apical GLUT2 switches to net secretion of accumulated glucose through apical GLUT2 into the lumen; it is then transported across the epithelium by a paracellular channel, along with other luminal glucose. Apical GLUT2 therefore acts, not as a direct transcellular transporter, but as a shunt to supply a major paracellular pathway of transepithelial absorption.
Paracellular flowThe proposal depends absolutely on the view that paracellular flow of glucose through tight junctions exists and, furthermore, that it occurs with rates comparable to observed luminal disappearance in perfusions in vivo. However, the concept of paracellular flow advanced by Pappenheimer lost favour during the debate with Diamond and Ferraris
22. In particular, they argued convincingly that paracellular markers, if they cross the intestinal barrier at all, do so only in low amounts after experiments of many hours or even longer, when the integrity of tissue becomes questionable. Even in cited example of cat, reported paracellular flow of glucose is only ~10% of glucose absorption. Thus the rate of absorption of paracellular markers is minimal to negligible compared with that for glucose.
Nevertheless, because of its potential importance, my laboratory made strenuous attempts to detect paracellular flow
12. Mannitol, the “gold standard” of paracellular markers was used, since L-glucose has a low affinity for SGLT1. Rats were maintained on a high carbohydrate diet and food was flushed from jejunum just before perfusion
in vivo; the perfusion was single pass at low flow rate and net glucose absorption (total absorption minus secretion) was determined chemically as luminal disappearance. At 75 mM luminal glucose, the steady-state rate of glucose absorption was established within just 15 min and was some 200-fold greater than for mannitol; see Table 1 of ref 12. “Closing” tight junctions by inhibiting myosin phosphorylation had no effect on mannitol clearance; nor did clearance change over a wide range of glucose concentrations at constant osmolarity (balanced with mannitol to 75 mM total sugar). Changes in water absorption correlated with changes in rapidly inserted apical GLUT2, not mannitol clearance.
Studies from other groups lead to same conclusion. In the work of Brot-Laroche and colleagues on apical GLUT2, fructose transport studies were done in vesicles
17; paracellular flow could not be involved. Apical GLUT2 mediated 60% of fructose transport, the rest was by GLUT5. The results with GLUT2 knockout mice correlated well with fructose and glucose perfusion data, one could be predicted from the other. Since GLUT5 and GLUT2 are facilitative transporters, there can be no concentration of the transcellular gradient
in vivo, yet fructose absorption at high luminal concentrations
in vivo shows the same apparent linearity as glucose
2.
Resistin
23 and metformin
24 promote AMP-mediated rapid insertion of apical GLUT2 in mice and rat to result in an increase in associated glucose absorption; there is no effect on minimal mannitol clearance. Notably, SGLT1 membrane density and ∆Isc were halved. This switch away from SGLT1 at high glucose concentrations was first observed in a study with the powerful AMP-kinase activator, AICAR, which induces a 3-fold increase in glucose absorption through a comparable increase in apical GLUT2
25. Simultaneously, SGLT1 is degraded to such an extent as to be almost undetectable (within ~30 min), so that only GLUT2 is at the apical membrane. The switch to the facilitative, energy-independent apical GLUT2 may represent a response to energy stress as the energy-dependent SGLT1 reaches maximal capacity.
Reports that paracellular flow is minimal to negligible under the conditions of apical GLUT2 studies have not been cited. Nor have any new experimental data been presented to change the debate on paracellular flow. The integrity of the intestinal barrier is paramount, as immunologists surely agree.
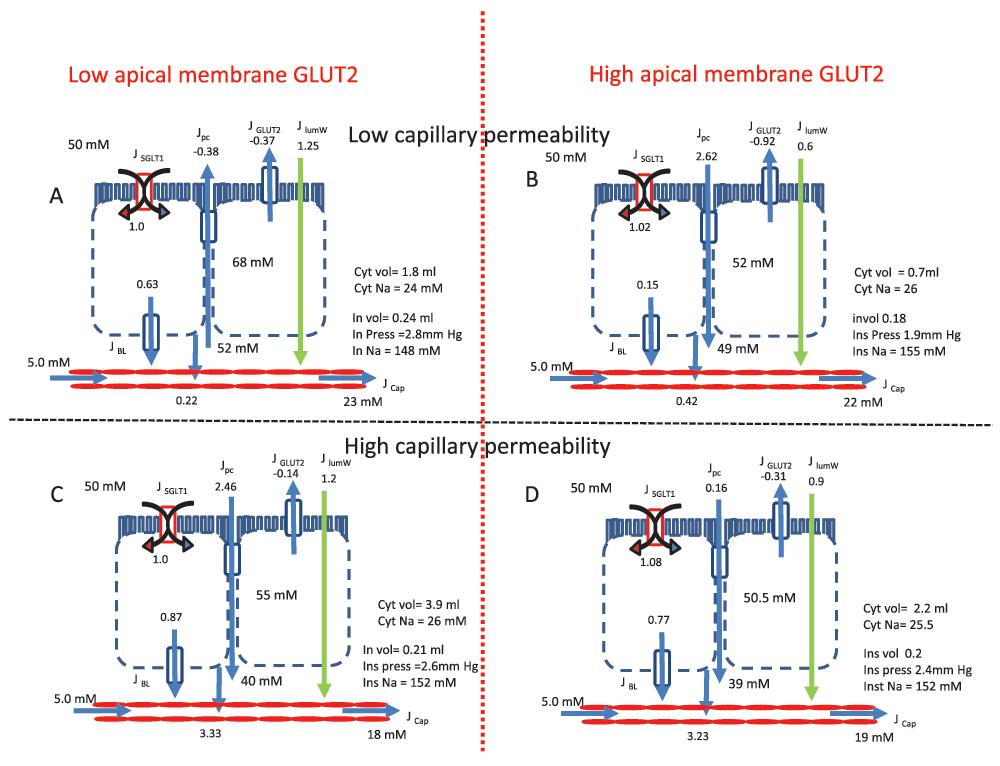
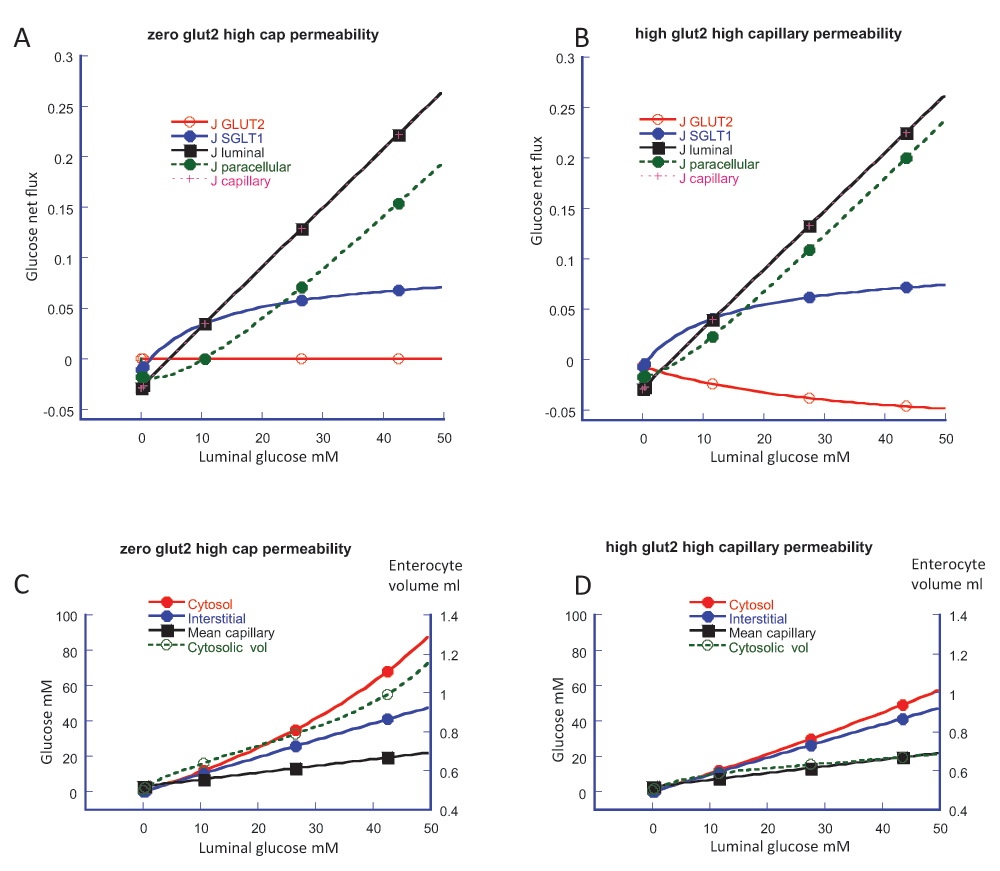
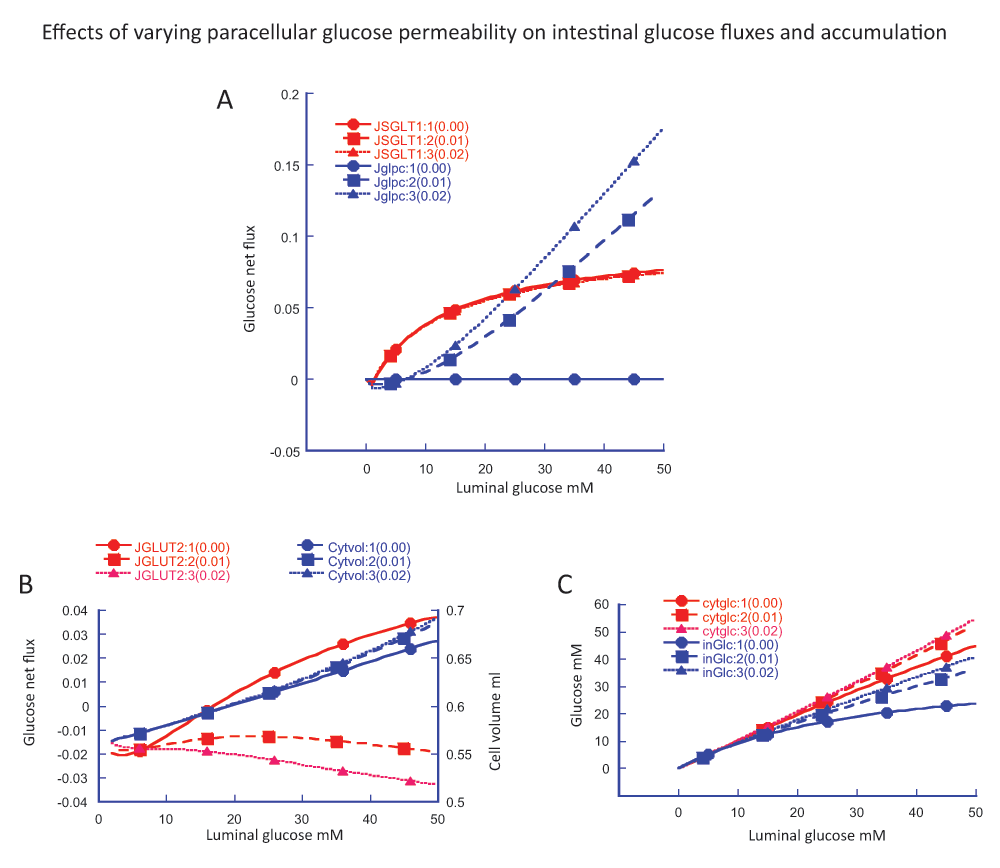
Intestinal transport is simulated with a computer-based modelThe novel paracellular flow proposal is based entirely on computer simulation of what is described as a “multiplicity and complexity of interactive processes”. As the extensive mathematical appendix so lucidly shows, simulation depends on many steps and their interactions, the choice of many parameters and their values, the concentrations of glucose and Na
+ at specific places within various compartments, and so on. Inevitably, a serious issue is that some, possibly most, values are of great uncertainty or simply unknown.
Two key examples suffice to make the point. The K
t of glucose for SGLT1 is taken as 17 mM, in contrast to reports of 23
26 and 26 mM
7 in vivo. The K
t of GLUT2 is also taken as 17 mM, reported for expression in oocytes. In contrast, the value of 56 ± 14 mM, based on an empirical sigmoid curve analysis of
in vivo data
7, is described as “very high”, implying the “diffusive” component is not GLUT2 but paracellular flow. In fact, the K
t of GLUT2 for uptake is 48 ± 5 mM for basolateral membrane vesicle preparations
27. Moreover, the question of asymmetry in this bidirectional transporter seems not to have been addressed: V
max for uptake is ~6-fold greater than for efflux
27. Such differences and uncertainties for most parameters surely have a considerable impact on simulation. Indeed, simply taking the K
t values of SGLT1 and GLUT2 to be the same as each other and much lower than the literature consensus strongly biases simulation outcome.
Transcellular Glucose Gradients In VivoIt is widely accepted that secondary active transport by SGLT1 results in glucose accumulation to enterocyte concentrations greater than in the lumen of intestinal preparations
in vitro. This is because the K
t for SGLT1 is very much lower than
in vivo (sub mM v 26 mM) and because preparations such as everted sacs have poor clearance. The question is whether glucose accumulation occurs
in vivo. If, as the balance of evidence indicates, there is negligible paracellular flow, then the transcellular gradient must be downhill (l-m-s) when luminal concentrations are high. Other evidence supporting this conclusion has been reviewed
3-5.
Recycling of Glucose and Na+ Across IntestineRecycling of certain key nutrients across the intestine is vital to its function. Thus Na
+ is recycled paracellularly to the lumen at high rates through claudin-15, to support continued activity of SGLT1 and other Na
+-dependent transporters
28. Knockout of claudin-15 results in abnormally low luminal Na
+ (~8 mM v ~57 mM for wild-type) and a large impairment in glucose absorption (∆Isc). Recycling of Na
+ is important when luminal glucose concentrations are low, e.g. between meals or overnight, in starvation and desquamation. Thus SGLT1 is the only transporter capable of driving glucose uphill against its gradient to plasma and therefore preventing loss of glucose by secretion through apical GLUT2.
Conversely, glucose is important for Na
+ recovery. A clear example is streptozocin-diabetes, which is characterised by hyperglycaemia and hyponatraemia; a permanently high level of apical GLUT2 is also found in diabetic rats
29. When luminal glucose is less than in plasma, secretion through apical GLUT2
16 is three-fold greater in diabetic than in normal rats and is increased by phloridzin
30. Thus recycling of glucose through apical GLUT2 is associated with recovery of Na
+ through SGLT1, providing potential compensation for loss of Na
+ by urinary excretion in diabetes. SGLT1 will also play a role in recovery of water secreted by apical GLUT2.
In the scenarios just outlined, there is clear physiological advantage to secretion of either Na
+ or glucose in their mutual recovery at low luminal concentrations. At high concentrations in the absence of paracellular flow, the switch to facilitated transcellular absorption through apical GLUT2 would be energy efficient and, as noted for periods of energy stress, might even be accompanied by a reduction of energy-dependent SGLT1. All that is required is a natural reversal of the transcellular concentration gradient in response to a fresh luminal glucose load. Energy-linked recycling of metabolic substrates used to be thought of as a waste of energy, a so-called “futile cycle.” It is now recognised as an amplification mechanism for rapid mobilisation of fresh substrate, or, by analogy, uptake of incoming glucose when absorption and secretion are initially comparable at low luminal glucose concentrations.
In addition to its roles as a transporter and in the mutual recovery of Na
+ and glucose, SGLT1 plays a major role in regulating apical GLUT2.
SUMMARY- This paper highlights the sheer complexity of developing an integrated model of intestinal glucose absorption. Such contributions to the literature are rare; they are valuable for forcing us to think about many things that we have yet to learn about, including apical GLUT2 and the in vivo situation, and to promote debate. Significant issues are inevitable, though, when such an ambitious objective is in mind.
- The novel model places paracellular flow at the heart of intestinal glucose absorption. However, new experimental evidence necessary to justify such a role is not presented. The account of the debate on paracellular flow is very selective and gives the erroneous impression that the concept is widely accepted. At best, it is controversial. A fuller account should be given.
- Specifically, recent papers from several groups report that paracellular flow is minimal under the conditions of apical GLUT2 studies, when large changes in absorption, apical GLUT2 and even SGLT1 are seen. Reinterpretation of apical GLUT2 data to support the novel paracellular flow model is not possible.
- Simulation results are presented for just one luminal glucose concentration (50 mM) and two rather selective values of Km for key transporters; there is no consideration of GLUT2 asymmetry. A much wider range of factors, concentrations and parameter values should be explored, including the absence of paracellular flow.
- There is significant literature evidence to support the view that, when the glucose concentration in the lumen is much higher than in plasma, the transcellular glucose gradient (l-m-s) is downhill all the way in vivo. The differences between in vivo and in vitro situations should be clarified.
- Emphasis on recycling of glucose through apical GLUT2 has been as a shunt to serve paracellular flow. Evidence that recycling of glucose and Na+ through SGLT1 is vital to intestinal function should be included.
- New title? “Computer simulation of an integrative model of intestinal glucose absorption”
1. Donhoffer S: Über die elektive resorption der zucker.
Arch Exp Pathol u Pharmakol. 1935;
177: 689-692
2. Pappenheimer JR, Reiss KZ: Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat.
J Membr Biol. 1987;
100 (2): 123-136
PubMed Abstract3. Kellett GL: The facilitated component of intestinal glucose absorption.
J Physiol. 2001;
531 (Pt 3): 585-595
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 4. Kellett GL, Brot-Laroche E: Apical GLUT2: a major pathway of intestinal sugar absorption.
Diabetes. 2005;
54 (10): 3056-3062
PubMed Abstract |
Publisher Full Text |
Reference Source 5. Kellett GL, Brot-Laroche E, Mace OJ, Leturgue A: Sugar absorption in the intestine: the role of GLUT2.
Annu Rev Nutr. 2008;
28: 35-54
PubMed Abstract |
Publisher Full Text |
Reference Source 6. Kellet GL: Alternative perspective on intestinal calcium absorption: proposed complementary actions of Ca(v)1.3 and TRPV6.
Nutr Rev. 2011;
69 (7): 347-370
PubMed Abstract |
Publisher Full Text 7. Kellett GL, Helliwell PA: The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane.
Biochem J. 2000;
15 (Pt 1): 155-162
PubMed Abstract |
Free Full Text |
Reference Source 8. Helliwell PA, Richardson M, Affleck J, Kellett GL: Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C.
Biochem J. 2000;
350 (Pt 1): 149-154
PubMed Abstract |
Free Full Text |
Reference Source 9. Helliwell PA, Richardson M, Affleck J, Kellett GL: Regulation of GLUT5, GLUT2 and intestinal brush-border fructose absorption by the extracellular signal-regulated kinase, p38 mitogen-activated kinase and phosphatidylinositol 3-kinase intracellular signalling pathways: implications for adaptation to diabetes.
Biochem J. 2000;
350 (Pt 1): 163-169
PubMed Abstract |
Free Full Text |
Reference Source 10. Helliwell PA, Kellet GL: The active and passive components of glucose absorption in rat jejunum under low and high perfusion stress.
J Physiol. 2002;
544 (Pt 2): 579-589
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 11. Helliwell PA, Rumsby MG, Kellet GL: Intestinal sugar absorption is regulated by phosphorylation and turnover of protein kinase C betaII mediated by phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent pathways.
J Biol Chem. 2003;
278 (31): 28644-28650
PubMed Abstract |
Publisher Full Text |
Reference Source 12. Mace OJ, Morgan EL, Affleck JA, Lister N, et al.: Calcium absorption by Cav1.3 induces terminal web myosin II phosphorylation and apical GLUT2 insertion in rat intestine.
J Physiol. 2007;
580 (Pt 2): 605-616
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 13. Morgan EL, Mace OJ, Affleck J, Kellett GL: Apical GLUT2 and Cav1.3: regulation of rat intestinal glucose and calcium absorption.
J Physiol. 2007;
580 (Pt 2): 593-604
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 14. Mace OJ, Affleck J, Patel N, Kellett GL: Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2.
J Physiol. 2007;
582 (Pt 1): 379-392
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 15. Mace OJ, Lister N, Morgan E, Shepherd E, et al.: An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine.
J Physiol. 2009;
587 (Pt 1): 195-210
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 16. Ait-Omar A, Monteriro-Supulveda M, Poitou C, Le Gall M, et al.: GLUT2 accumulation in enterocyte apical and intracellular membranes: a study in morbidly obese human subjects and ob/ob and high fat-fed mice.
Diabetes. 2011;
60 (10): 2598-2607
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 17. Gouyon F, Caillaud L, Carriere V, Klein C, et al.: Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: A study in GLUT2-null mice.
J Physiol. 2003;
552 (Pt 3): 823-832
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 18. Tobin V, Le Gall M, Fioramonti X, Stolarczyk E, et al.: Insulin internalizes GLUT2 in the enterocytes of healthy but not insulin-resistant mice.
Diabetes. 2008;
57 (3): 555-562
PubMed Abstract |
Publisher Full Text |
Reference Source 19. Gorboulev V, Schurmann A, Vallon V, Kipp H, et al.: Na+-D-glucose Cotransporter SGLT1 is Pivotal for Intestinal Glucose Absorption and Glucose-Dependent Incretin Secretion.
Diabetes. 2012;
61 (1): 187-196
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 20. Kellett GL: Comment on: Gorboulev et al. Na+-D-glucose cotransporter SGLT1 Is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion.
Diabetes. 2012;
61 (6): e4
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 21. Roder PV, Geillinger KE, Zietek TS, Thorens B, et al.: The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing.
PLoS One. 2014;
9 (2): e89977
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 22. Ferraris RP, Diamond J: Regulation of intestinal sugar transport.
Physiol Rev. 1997;
77 (1): 257-302
PubMed Abstract |
Reference Source 23. Krimi RB, Letteron P, Chedid P, Nazaret C, et al.: Resistin-like molecule-beta inhibits SGLT-1 activity and enhances GLUT2-dependent jejunal glucose transport.
Diabetes. 2009;
58 (9): 2032-2038
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 24. Sakar Y, Meddah B, Faouzi MA, Cherrah Y, et al.: Metformin-induced regulation of the intestinal D-glucose transporters.
J Physiol Pharmacol. 2010;
61 (3): 301-307
PubMed Abstract |
Reference Source 25. Walker J, Jijon HB, Diaz H, Salehi P, et al.: 5-aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK.
Biochem J. 2005;
385 (Pt 2): 485-491
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 26. Debnam ES, Levin RJ: An experimental method of identifying and quantifying the active transfer electrogenic component from the diffusive component during sugar absorption measured in vivo.
J Physiol. 1975;
246 (1): 181-196
PubMed Abstract |
Free Full Text |
Publisher Full Text |
Reference Source 27. Please write authors as Surname X, Surname Y: The Na+-independent D-glucose transporter in the enterocyte basolateral membrane: orientation and cytochalasin B binding characteristics.
J Membr Biol. 1987;
97 (3): 259-266
PubMed Abstract28. Tamura A, Hayashi H, Imasato M, Yamazaki Y, et al.: Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine.
Gastroenterology. 2011;
140 (3): 913-923
PubMed Abstract |
Publisher Full Text |
Reference Source 29. Corpe CP, Basaleh MM, Affleck J, Gould G, et al.: The regulation of GLUT5 and GLUT2 activity in the adaptation of intestinal brush-border fructose transport in diabetes.
Pflugers Arch. 1996;
432 (2): 192-201
PubMed Abstract30. Levine GM, Shiau YF, Deren JA: Characteristics of intestinal glucose secretion in normal and diabetic rats.
Am J Physiol. 1982;
242 (5): G455-G459
PubMed Abstract |
Reference Source No competing interests were disclosed.


Comments on this article Comments (0)