Case presentation
This case involves a 13 year-old Caucasian boy with a history of cystic fibrosis status post deceased donor EnBloc combined liver and pancreas transplantation, presenting with gastrointestinal bleeding. His immune suppression regimen after the transplant included tacrolimus 1.5 mg twice daily.
Two years after his transplant, the patient presented to the hospital with “a few days of dark tarry stool”. He did not complain of any nausea, vomiting, or abdominal pain. He denied taking any anti-platelet agents or non-steroidal inflammatory drugs. On admission, the patient was hypotensive with a blood pressure of 70/50 mmHg and tachycardic with a heart rate of 112 beats per minute. His hemoglobin was 7.4 g/dL, which represented a drop from a baseline hemoglobin of 10 g/dL. After fluid resuscitation, the patient was started on pantoprazole continuous infusion at 5 mg/hour and sulcrafate suspension 300 mg four times daily. He then underwent an urgent upper endoscopy, which revealed multiple small prepyloric and duodenal ulcerations without signs of recent hemorrhage. Biopsies of these ulcers showed acute inflammation. A capsule endoscopy was then performed, which showed scattered duodenal erosions and two adjacent erosions in the distal duodenum without stigmata of recent bleeding. During the work-up, the patient continued to have melena, necessitating transfusions every three days. This prompted a second upper endoscopy, which showed complete healing of the prior ulcers. Interestingly, portal hypertensive gastropathy and a gastric varix were also noted. To target bleeding lesions potentially related to portal hypertension, octreotide continuous infusion at 2 mcg/kg/hour was initiated. Unfortunately, his melena persisted. The patient underwent a technetium-labeled red blood cell bleeding scan, where a potential bleeding source “near the anastomosis of the native to transplanted duodenum or proximal jejunum” was identified. A visceral arteriogram was obtained but it failed to detect a bleeding source. Following these radiological tests, a deep enteroscopy was performed. No signs of recent bleed within the suspected proximal hepatic limb of the Roux were detected. In the jejunum, however, there was a 10 mm clean-base, friable ulcer with significant oozing when it was biopsied. The pathological diagnosis of the ulcer was reported as follows: “the morphologic and immunophenotypic features indicate involvement of this patient’s jejunum of a large B-cell lymphoproliferative disorder consistent with diffuse large B-cell lymphoma (DLBCL). These findings thus support the diagnosis of a post-transplantation lymphoid disorder, involving small intestinal mucosa”. Epstein-Barr virus (EBV) was positive in the biopsied sample (See Figure 1).
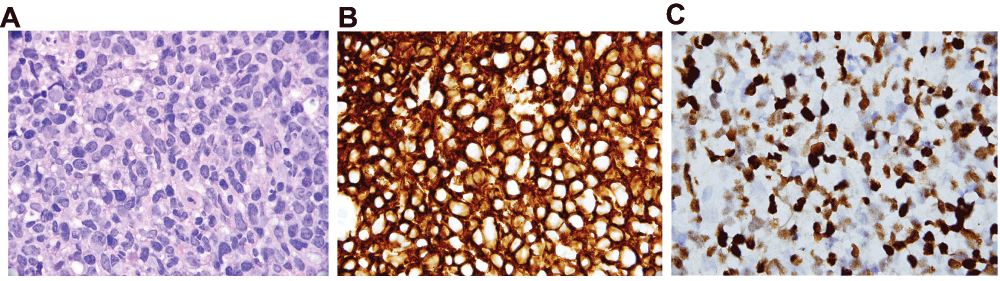
Figure 1. Biopsy of jejunal ulcer.
1A: Haematoxylin & Eosin stain, 1000x. Large, centroblastic lymphoid cells with hyperchromatic, pleomorphic nuclei and moderate cytoplasm. These tumor cells almost completely efface the jejunal mucosa. Scattered mitotic activity and apopotic debris are present as well. 1B: Immunohistochemical stain for CD20, 1000x. Diffusely positive membranous staining in the tumor cells is indicative of lymphoid differentiation. In the context of the morphology seen in Figure 1A, the findings are compatible with a Diffuse Large B-Cell Lymphoma (DLBCL). Per the 2008 World Health Organization (WHO) classification, a lymphoid proliferation that meets the criteria for a high grade malignancy (in this case DLBCL) that occurs in a recipient of a solid organ, bone marrow, or stem cell transplant is diagnostic of a post-transplant lymphoproliferative disorder (PTLD). 1C: In situ hybridization for Epstein-Barr virus encoded RNA (EBER), 1000x. Strong nuclear positivity is seen in the majority of the tumor cells, a finding seen in many monomorphic PTLD cases.
Follow-up and outcome
An abdominal MRI performed a few days later demonstrated a mild thickening of the segments of small bowel in the left upper quadrant and portal hypertension. No lymphadenopathy was found in the abdomen and pelvis. The patient was then evaluated by the pediatric oncology service. A bone marrow biopsy reassuringly showed a normocellular marrow. The patient was started on cyclophosphamide, rituximab, and prednisone. His gastrointestinal bleeding eventually stopped a few weeks later.
Discussion
PTLD are rare but potentially fatal complications of solid organ transplantation3. The overall incidence of lymphoproliferative disorders in the transplant population is approximately 1% at 10 years, which is significantly higher than the general population3. The risk increases with the intensity of induction or rescue immunosuppression, and particularly following monoclonal or polyclonal anti-lymphocyte therapy4–6. The EBV serostatus of the recipient is another risk factor7, although PTLD can occur in EBV-negative diseases as well. Time post-transplant is also a key factor as the majority of PTLD occurs within the first year post-transplant8. Over half of the patients with PTLD present with extranodal masses9. PTLD can affect many organs, including the gastrointestinal tract, lungs, liver, central nervous system, and the allograft itself. Clinical manifestations vary, ranging from benign polyclonal lymphoproliferation (infectious mononucleosis-type acute illness) to aggressive and disseminated malignant disease. Gastrointestinal features typically include abdominal pain, fever, and bowel perforation in serious cases. There have been case reports describing gastrointestinal bleeding as the initial presentation of PTLD in the pediatric population10–13, but this is less common. This case highlights the importance of considering the diagnosis of post-transplantation lymphoproliferative disorders in transplant receipts presenting with unexplained gastrointestinal hemorrhage.
Consent
Informed consent for publication of clinical details and clinical images was obtained from the next of kin.
Author contributions
EH: acquisition of data, interpretation of data, drafting of manuscript, assistance in clinical care.
VG: preparation and processing of pathology slides, interpretation of data.
MM, JO: acquisition of data, interpretation of data, guidance of clinical management plan, revision of manuscript.
Competing interests
No competing interests were disclosed.
Grant information
The author(s) declared that no grants were involved in supporting this work.
Faculty Opinions recommendedReferences
- 1.
Penn I:
Cancers complicating organ transplantation.
N Engl J Med.
1990; 323(25): 1767–1769. PubMed Abstract
| Publisher Full Text
- 2.
Adami J, Gäbel H, Lindelöf B, et al.:
Cancer risk following organ transplantation: a nationwide cohort study in Sweden.
Br J Cancer.
2003; 89(7): 1221–1227. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 3.
Curtis RE, Travis LB, Rowlings PA, et al.:
Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study.
Blood.
1999; 94(7): 2208–2216. PubMed Abstract
- 4.
Feldman M, Friedman LS, Brandt LJ:
Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology / Diagnosis / Management. Volume 2. 9th edition, Canada: Saunders Elsevier; 2010. Reference Source
- 5.
Jamali FR, Otrock ZK, Soweid AM, et al.:
An overview of the pathogenesis and natural history of post-transplant T-cell lymphoma (corrected and republished article originally printed in Leukemia & Lymphoma, June 2007; 48(6): 1237–1241).
Leuk Lymphoma.
2007; 48(9): 1780–1784. PubMed Abstract
| Publisher Full Text
- 6.
Allen U, Hébert D, Moore D, et al.:
Epstein-Barr virus-related post-transplant lymphoproliferative disease in solid organ transplant recipients, 1988–97: a Canadian multi-centre experience.
Pediatr Transplant.
2001; 5(3): 198–203. PubMed Abstract
| Publisher Full Text
- 7.
Walker RC, Marshall WF, Strickler JG, et al.:
Pretransplantation assessment of the risk of lymphoproliferative disorder.
Clin Infect Dis.
1995; 20(5): 1346–1353. PubMed Abstract
| Publisher Full Text
- 8.
Opelz G, Henderson R:
Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients.
Lancet.
1993; 342(8886–8887): 1514–1516. PubMed Abstract
| Publisher Full Text
- 9.
Nalesnik MA, Jaffe R, Starzl TE, et al.:
The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression.
Am J Pathol.
1988; 133(1): 173–192. PubMed Abstract
| Free Full Text
- 10.
Lai YC, Ni YH, Jou ST, et al.:
Post-transplantation lymphoproliferative disorders localizing to the gastrointestinal tract after liver transplantation: report of five pediatric cases.
Pediatr Transplant.
2006; 10(3): 390–394. PubMed Abstract
| Publisher Full Text
- 11.
Heo JS, Park JW, Lee KW, et al.:
Posttransplantation lymphoproliferative disorder in pediatric liver transplantation.
Transplant Proc.
2004; 36(8): 2307–2308. PubMed Abstract
| Publisher Full Text
- 12.
Donnelly LF, Frush DP, Marshall KW, et al.:
Lymphoproliferative disorders: CT findings in immunocompromised children.
AJR Am J Roentgenol.
1998; 171(3): 725–731. PubMed Abstract
| Publisher Full Text
- 13.
Lim GY, Newman B, Kurland G, et al.:
Posttransplantation lymphoproliferative disorder: manifestations in pediatric thoracic organ recipients.
Radiology.
2002; 222(3): 699–708. PubMed Abstract
| Publisher Full Text

Comments on this article Comments (0)