Keywords
Sepsis, Trials, Septic Shock,
Sepsis, Trials, Septic Shock,
The global burden of critical care1 is increasing rapidly for many reasons, one of which is the increasing incidence and prevalence of sepsis. Sepsis is a common cause of admission to an Intensive Care Unit (ICU) and the commonest cause of death in ICU. Interestingly, despite no new drug approvals for sepsis since activated protein C (APC) (and the withdrawal of APC from the world market), sepsis mortality appears to be decreasing over the last decade2 from about 40% to about 20%. Most attribute this success to the improved process of care, including the earlier identification of sepsis in the Emergency Department and earlier initiation of appropriate antibiotics, fluid and vasopressor resuscitation, and low tidal volume during mechanical ventilation3–8. The decreasing mortality, by definition, leads to increasing numbers of survivors of severe sepsis and septic shock. Thus, the long-term outcome and morbidity of survivors is an emerging, centrally important research and clinical care concern in sepsis.
A very recent, very large (n = 1,171,197!) cohort study evaluated whether the numbers of systemic inflammatory response syndrome (SIRS) criteria a patient has when presenting with sepsis is associated with increased mortality9. The vast majority (88%) had SIRS-positive severe sepsis whereas only about 12% had SIRS-negative severe sepsis. Interestingly, the declines in mortality rates described above were similar in these two groups, SISR positive and SIRS negative. The authors therefore conclude that SIRS criteria to define severe sepsis may not be helpful or useful because the usual definition of needing two or more SIRS criteria to define severe sepsis excluded about 12% of patients with severe sepsis. However, in the SIRS-positive group, mortality increased as the number of SIRS criteria was positive, suggesting that the sum of SIRS criteria does have prognostic value. This will create more controversy because a growing number of authors have questioned the value and need for SIRS criteria to define sepsis and severe sepsis.
It is important to highlight that the parallel increase in the incidence of severe sepsis (and septic shock) worldwide and the reduction in its mortality rate may have another “administrative” explanation and that the changes may not be as real initially considered. Several investigators10,11 have argued that the higher incidence of reported cases of “mild sepsis” is due to greater awareness of the problem (and the sepsis definition) as well as enhanced reimbursement for a sepsis diagnosis, thus incentivizing the diagnosis of sepsis definition). Ironically, while the incidence of organ-specific infections remains fairly constant over the last decade, the incidence of severe sepsis continues to increase10. And perhaps even more important, the greater inclusion of mild cases under the umbrella of “severe sepsis”, which were less often reported in the past, may artificially decrease the pooled mortality rate of sepsis in cohorts and administrative databases. These observations challenge partially the statement that the mortality of severe sepsis has decreased, although there could also be a true decrease of mortality too.
What about the mortality rates of randomized controlled trials of different eras? Why have those mortality rates also been decreasing? Perhaps the patient populations of severe sepsis included in “old” RCTs differ from more recent RCTs. Even in the more recent RCTs, there is a marked discrepancy between mortality rates of European trials compared to North American and Australian RCTs. One possible explanation is that baseline characteristics and risks of death of the study populations enrolled differ across continents and jurisdictions. In support of this hypothesis, the incidence of mechanical support (which may be a surrogate of the overall severity and risk of death) at the time of study enrollment is about 85–90% of the study populations in European RCTs, versus only about 40–50% in the American and Australian trials. VASST12 (vasopressin vs. norepinephrine in septic shock) and CORTICUS13 (European RCT of corticosteroids vs. placebo in septic shock) were reported in 2008 and both had an incidence of mechanical ventilation of about 85–90%. Thus, it is difficult to compare RCTs of different continents and of different eras as there are uncontrolled differences and changes over time in processes of care (and that could improve outcomes) as well as changes in populations included (and that could alter risk of death).
As the mortality of severe sepsis and septic shock continues to decrease, the number of survivors is increasing steadily; however, this is not all good news. Long-term outcome is impaired in 1-year survivors in the ensuing 1–10 year period after septic shock, even after adjusting for underlying co-morbidities14. Furthermore, even mild stage 1 AKI is significantly associated with impaired long-term (10 years) outcome of septic shock15. Cognitive dysfunction, neuromuscular weakness and neuromyopathies, post-traumatic stress disorder (PTSD), and depression are common long-term complications of septic shock16. Cognitive and functional decline, in part, reflect pre-existing declines in those domains that may place such patients at risk for sepsis17.
Most RCTs have been negative in sepsis and, in particular, no new therapies for modification of the host response have been successfully introduced into clinical practice. Recent examples include eritoran18, and polyclonal anti-tumor necrosis factor (TNF)19. Eritoran is an inhibitor of the early innate immune response that signals via Toll-Like receptor 4 (TLR4) and its associated molecule, MD2. Eritoran did not alter mortality or secondary outcomes in 1,961 severe sepsis patients. Similarly, anti-TNF did not display an efficacy signal in a Phase 2b multicenter RCT. Talactoferrin actually increased mortality (http://www.genengnews.com/gen-news-highlights/agennix-stops-phase-ii-iii-talactoferrin-study-in-sepsis-due-to-higher-death-rates/81246312), despite a seemingly positive Phase 2 RCT20. Accordingly, new RCT designs are needed and new approaches to sepsis therapy must be considered.
New RCT designs are needed in sepsis21. Three changes could increase the chances of having positive RCTs in sepsis: the use of biomarkers, response adaptive trial design, and composite organ dysfunction endpoints.
Predictive biomarkers define subgroups of septic patients who have an enhanced (or adverse) response to a specific treatment. The use of predictive biomarkers (such as Her2neu for Herceptin use) has revolutionized cancer RCTs, and clinical drug protocols leading to improved outcomes of many cancers. Furthermore, biomarkers could improve patient selection through more accurate diagnosis of sepsis. Finally, outcomes of sepsis vary widely depending on the source of sepsis22, so perhaps future sepsis RCTs should stratify by the source of sepsis or only include particular sources of sepsis (e.g., severe community-acquired pneumonia) in RCTs in sepsis.
Response-adaptive trial design can improve the efficiency of sepsis RCTs by optimizing dose selection and by stopping futile trials earlier because complex adaptive trial design makes use of interim data in nearly real time to adjust randomization and to select optimal dosing prior to completion. While industry is increasingly using adaptive trial design, academia has been slower to adopt this method. It is difficult to design outcomes in sepsis phase 2 trials due to the competing risk of death. Therefore, current phase 2 trials may not be predictive of success in phase 3 trials and the response-adaptive trials could help improve this situation.
The many failed RCTs of therapeutics for sepsis used 30-day mortality as the primary endpoint. The usual primary sepsis trial endpoint of 28 or 30-day mortality is roughly 6–8% lower than 90 day mortality in recent RCTs of sepsis. This observation, along with the prevalence of impaired long-term outcomes beyond 90 days, suggests that the “standard” 28-day mortality outcome in sepsis trial is an inadequate measure of the burden of sepsis. The recent RCTs (ProCESS23, ARISE24, ProMISe25 and Cohort studies2 show that the mortality rates of sepsis are lower than in RCTs published in 2008 of vasopressin versus norepinephrine12 (mortality rate 35–39%) and corticosteroids versus placebo13 (mortality rate 36–39%) (Table 1 and Table 2).
Accordingly, there are greater numbers of sepsis survivors, many of whom have ongoing morbidities and increased long-term (10-year) mortality rates. Therefore, it is appropriate and timely to adjust to the use of composite endpoints in sepsis RCTs. Examples of composite outcomes could include days alive and free of vasopressors and ventilation (for a drug that modified cardiovascular and pulmonary dysfunction) or days alive and free of vasopressors, ventilation and renal replacement therapy (for a drug that modulated these three organ dysfunctions). Composite cardiovascular endpoints have been the norm in academic and industry cardiovascular RCTs for several decades. As discussed under the section long-term outcomes of sepsis, such outcomes are also of value for therapies that could have such sustained benefits.
We also note that another critical strategy to improve the likelihood of future RCTs in severe sepsis and septic shock being positive depends upon on a better understanding of the molecular and pathophysiological rationale upon which we may decide to test, or not, a specific drug or device. Furthermore, the field is now widely aware that acute short-term models in healthy animals are poor models of human severe sepsis and septic shock. It is likely that the reason for some examples of negative RCTs in severe sepsis and septic shock is an incompletely clear molecular and pathophysiological rationale based on inadequate pre-clinical models.
| Trial (citation number) | Treatment N | Control N | Control N |
|---|---|---|---|
| Yealy23 (ProCESS) | EGDT 439 | Protocol-directed 446 | Usual care 456 |
| Peake24 (ARISE) | EGDT 793 | Usual care 798 | |
| Mouncey25 (ProMISe) | EGDT 630 | Usual care 630 | |
| Asfar38 | High map 388 | Low map 388 | |
| Caironi40,46 (ALBIOS) | Albumin 903 | Crystalloid 907 | |
| Holst43 | Hg <7 RBCs 502 | Hg <9 RBCs 496 | |
| Lacroix44 | Fresh RBCs 1211 | Standard-issue RBCs 1219 | |
| Harvey45 | Parenteral nutrition 1191 | Enteral nutrition 1197 | |
| Mcauley31 | Simvastatin 259 | Placebo 280 | |
| Truwit32 | Rosuvastatin 379 | Placebo 366 | |
| Totals | 6,645 | 6,637 | 456 |
| Grand total = 13,282 |
| Trial (mortality time point) | Treatment Mortality rate (%) | Control Mortality rate (%) | Control Mortality rate (%) | P |
|---|---|---|---|---|
| Yealy (ProCESS) (day 60) | EGDT 21 | Protocol-directed 18.2 | Usual care 18.9 | 0.83 |
| Peake24 (ARISE) (day 90) | EGDT 18.6 | Usual care 18.8 | 0.98 | |
| Mouncey25 (ProMISe) 90 day | EGDT 29.5 | Usual care 29.2 | 0.9 | |
| Asfar38 (day 28) | High map 36.6 | Low map 34 | 0.57 | |
| Caironi40,46 (ALBIOS) (day 28) | Albumin 31.8 | Crystalloid 32 | 1.0 | |
| Holst43 (day 90) | Hg <7 RBCs 43 | Hg <9 RBCs 45 | 0.94 | |
| Lacroix44 | Fresh RBCs 37 | Standard RBCs 35.3 | 0.38 | |
| Harvey45 (day 30) | Parenteral nutrition 33.1 | Enteral nutrition 34.2 | 0.57 | |
| Mcauley31 (day 28) | Simvastatin 22 | Placebo 26.8 | 0.23 | |
| Truwit32 (day 60) | Rosuvastatin 28.5 | Placebo 24.9 | 0.21 |
There were a number of large multicenter RCTs published in 2014 and early 2015 that are directly or indirectly concerned with severe sepsis and septic shock (Table 1 and Table 2). The eight RCTs highlighted in Table 1 and Table 2 recruited a total of 13,282 critically ill patients, a remarkably large number. Unfortunately, none of these RCTs achieved pre-specified statistically significant results on their respective primary endpoints!
The majority of failed clinical RCTs of novel biologics for treatment of sepsis share key features. First, most of the trials aimed to modulate the host response to infection. Modulation of the host response to sepsis may have failed, in part, because the host septic inflammatory response is complex, involving many parallel redundant biological pathways that are expressed to a lesser or greater extent at different times, in different tissues, in different patients26. Furthermore, a number of the pathways are detrimental in some patients and therefore could reasonably be inhibited (for example, TNFα detrimentally drives a systemic inflammatory response) but not in other patients (TNFα helps wall off focal infection, such as localized bowel perforation). Second, enrollment in these RCTs was delayed by the process of identifying sepsis, by the processes of obtaining informed consent and delivering the therapeutic to the patient; thus, often, the novel therapeutics were delivered to the patient 12 hours or even more than 72 hours after the onset of sepsis. A key lesson learned from early EGDT studies, and by the more recent ProCESS23 and ARISE24 trials, is that early identification and treatment, notably including early antibiotics, profoundly reduces mortality from sepsis, severe sepsis, and septic shock27.
Accordingly, the search for novel therapeutics to treat sepsis should be cautious regarding the modulation of the late host response and, instead, focus on the earliest aspects of sepsis—particularly the pathogen and clearance of pathogen-derived products—that initially trigger the host inflammatory response.
Proprotein convertase subtilisin/kexin type-9 (PCSK9) is an exciting candidate for treatment because anti-PCSK9 strategies target the organism(s), not the host response (a strategy that has almost uniformly failed)28 (Figure 1). The potential benefit of this approach arises from an understanding of the normal clearance pathways of sepsis-initiating pathogen molecules. The innate immune system provides the earliest response to infecting pathogens when pathogen-associated molecular patterns (PAMPs) bind and activate innate immune receptors, such as Toll-Like receptors. Key PAMPs from bacteria are lipid-containing molecules that arise from bacterial cell walls, such as lipopolysaccharide (LPS) from Gram-negative bacteria, or lipotechoic acid from Gram-positive bacteria. To clear these pathogen lipids from the circulation of septic patients, these molecules are first bound by transfer proteins, such as LPS-binding protein (LBP) and bactericidal/permeability-inducing protein (BPI). These transfer proteins are highly homologous to other lipid-carrying transfer proteins, such as phospholipid transfer protein (PLTP) and cholesterol ester transfer protein (CETP), molecules that are much more familiar to cardiologists and others caring for patients with hypercholesterolemia and atherosclerotic cardiovascular disease. Like PLTP and CETP, the pathogen lipid transfer proteins are carried within, and equilibrate between, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low density lipoprotein (VLDL)27. Then, pathogen lipids are cleared by the liver via the LDL receptor expressed on hepatocytes and by other less clearly identified routes.
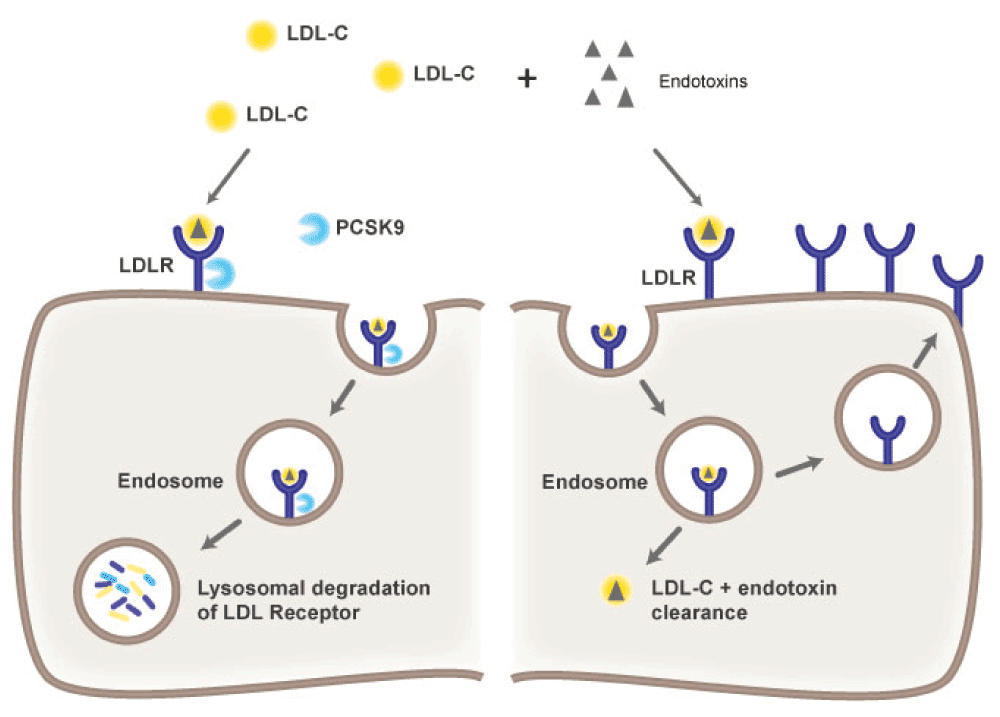
Pathogen lipids, such as endotoxins from Gram-negative bacteria and lipotechoic acid from Gram-positive bacteria, are carried within high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL) particles in the blood. Pathogen lipids contained within LDL cholesterol (LDL-C) are then cleared via LDL receptors (LDLR) expressed on hepatocytes. The left hand panel illustrates the role that proprotein convertase subtilisin/kexin type 9 (PCSK9) plays in modulating clearance. PCSK9 binds the LDLR and when LDLR is internalized, as part of hepatocyte uptake of LDL-C, bound PCSK9 targets the LDLR for lysosomal degradation. As a result, LDLR density on the hepatocyte cell surface diminishes. The right hand panel shows that when PCSK9 concentrations are reduced, LDLR density increases so that the clearance of LDL-C is increased. In the setting of sepsis, when LDL-C carries pathogen lipids, increased LDLR density also results in the increased clearance of pathogen lipids, leading to a diminished inflammatory response and improved sepsis outcomes.
The LDL receptor is thus a key step in the clearance of pathogen lipids from the circulation in sepsis, severe sepsis and septic shock28. LDL particles bind to the LDL receptor and, with the LDL receptor, are internalized within the hepatocyte (Figure 1). When cleared of LDL, the receptor is then recirculated to the hepatocyte cell surface. As recently described in the cardiovascular literature30, the number of LDL receptors expressed on hepatocytes is regulated by PCSK9. PCSK9 in the circulation binds LDL receptors on hepatocytes. When the LDL receptor is internalized, bound PCSK9 targets the LDL receptor for lysosomal degradation. This results in decreased LDL receptor density on hepatocytes that, in turn, reduces LDL receptor-mediated clearance of LDL (of interest to physicians treating atherosclerosis risk) but also reduces the clearance of pathogen lipids (of great interest in sepsis). Conversely, pharmaceuticals or genetic variations that reduce PCSK9 function, increase the expression of the LDL receptor on hepatocytes, and thereby increase the clearance of LDL from the blood28.
Accordingly, knockout of the PCSK9 in mice reduces the adverse systemic inflammatory response when LPS is administered, by increasing the clearance rate of LPS28. Inhibition of PCSK9 in a model of polymicrobial septic peritonitis decreases the inflammatory cytokine response and results in increased survival. The beneficial effect of decreased PCSK9 function also appears to have an important effect in human sepsis. Septic shock patients carrying one or more loss-of-function genetic variants of the PCSK9 gene have significantly improved outcomes: decreased inflammatory cytokine response and decreased 28-day mortality. Conversely, septic shock patients carrying gain-of-function PCSK9 genetic variants have adverse outcomes: increased septic inflammatory cytokine response and increased mortality. This raises the hypothesis that pharmacologic inhibition of PCSK9 may improve outcomes in septic patients by enhancing the clearance of pathogen lipids by the liver, thereby decreasing the cytokine inflammatory response and its physiologic and clinical phenotype consequences28.
If PCSK9 inhibition (conceptually the new “super statin”) improves the outcome in sepsis, then does conventional statin therapy also improve outcomes from sepsis and related organ dysfunction, such as ARDS? After a number of days of treatment, statins indirectly increase LDL receptor expression as a consequence of the statin-induced decrease in LDL. This should be beneficial in sepsis. However, statin treatment also indirectly increases PCSK9 concentrations, again as a response to decreased LDL. Thus, the net effect of statins was not known. Simvastatin31 and rosuvastatin32 did not alter outcomes of ARDS, the commonest organ dysfunction associated with sepsis. Perhaps it would be logical to test earlier statins and more adequate plasma statin levels during treatment in future RCTs in sepsis. However, PCSK9 inhibition may also be required to counteract the adverse impact of statin-induced elevations of PCSK9 levels. The Papazian study33 of simvastatin for ventilator-associated pneumonia (VAP) was stopped early for futility with a hazard ratio of 1.45 [95% CI, 0.83 to 2.51].
Early protocolized care of septic patients has been almost universally adopted and has transformed patient outcomes2. One major contributor to improved outcomes appears to be earlier identification of sepsis by physician and non-physician Emergency Department (ED) staff, facilitated by the implementation of manual or computerized algorithms. Earlier identification sepsis algorithms also drive the early administration of appropriate antibiotics and that appears to be a key contributor. Another contributor may be early fluid resuscitation and hemodynamic support. The ProCESS23, ARISE24 and ProMISe25 trials sought to identify the key beneficial aspects of Rivers’ early EGDT34 in the current 21st century ED environment, where early identification, antibiotics, and fluid resuscitation are considered usual care. In view of the current ED environment, these studies did not achieve very substantial clinical differences in resuscitation (processes of care) between treatment groups, and all patients had received many aspects of Rivers’ EGDT and had achieved many of the EGDT targets by the time of enrollment into ProCESS and ARISE (e.g., initial mean central venous oxygen saturation in ProCESS was above the EGDT target of >70%, mean Central venous oxygen saturation [ScvO2] was about 65% in ProMISe25). Enrollment in ProCESS and ARISE sometimes took substantially longer (up to 12 hours) compared to Rivers’ EGDT (mean 1.5 hours) so that these later RCTs may have interrogated patients somewhat later and after more substantial resuscitation, and thus improvement. ProMISe recruited a mean of 2.5 hours after meeting inclusion criteria.
The ProCESS23 and ARISE24 RCTs had low pooled mortality rates (18–22%) and there was no difference in mortality between EGDT and usual care groups (Table 2). ProMISe used 90-day mortality that was as expected higher (pooled mortality 29%), with no difference between EGDT and usual care groups (29.5 vs. 29.2% respectively; Table 2).
It is challenging to arrive at firm conclusions for RCTs with minimal differences in processes of care between the treatment groups and with no significant difference in outcomes. Nevertheless, the low mortality rates of the control groups in ProCESS and ARISE suggest that many changes in care (e.g., early identification of sepsis, early administration of antibiotics and early, effective source control) have decreased the mortality rate of severe sepsis. The negative results of ProCESS and ARISE suggest that targeting a central venous saturation greater than 70% is one of several effective approaches to determining whether fluid and vasopressor resuscitation adequately reversed hypoperfusion. For example, ProCESS allowed investigators to rely on clinical judgment which, in this setting, appeared to be as good as EGDT guided care, while Jones et al.35 suggest that lactate clearance is as good a target as central venous oxygen saturation targeting. Indeed, Rivers’ EGDT implies that a low mixed venous oxygen saturation (after adequate fluid and vasopressor resuscitation, in the setting of adequate blood oxygen carrying capacity) raises the possibility of impaired cardiac function. With the recent emergence of routine goal-directed echocardiography in the ED, the diagnosis of impaired cardiac function need not await measurements of central venous oxygen saturation36.
The optimal mean arterial blood pressure (MAP) for septic shock patients is unknown. Higher arterial pressures may be more effective at perfusing some high-resistance vascular beds in vital organs, such as the brain, kidneys and heart, yet higher MAP targets may require greater use of vasopressors with potential adverse consequences. Asfar and colleagues37 compared a high MAP (80–85 mmHg) with a lower MAP (65–70 mmHg) over the course of 5 days, or until cessation of vasopressor infusions in septic shock patients38. They found no overall difference in mortality rates between high and low target MAP. As expected, the high MAP target was associated with increased vasopressor use and, consequently, with an increased incidence of dysrhythmias (especially atrial fibrillation). However, in the a priori subgroup of patients who had pre-existing hypertension, patients randomized to the high MAP group had significantly lower incidences of AKI and need for RRT (32% versus 42%, unadjusted P=0.046)39.
Whether the choice of fluid for septic shock resuscitation alters outcome continues to be debated. ALBIOS40 showed no difference in mortality between albumin resuscitation and maintenance of a serum albumin >30 G/L while in ICU for patients with severe sepsis/septic shock, compared to usual care with crystalloid resuscitation. In a post hoc analysis, there was a lower mortality in the albumin compared to the crystalloid resuscitation group in the subgroup of patients who had septic shock. Optimal choice of fluids from resuscitation, therefore, remains an open issue.
The pivotal Transfusion Requirements in Critical Care (TRICC) RCT41 found that a conservative transfusion strategy (to transfuse when Hg <7 g/dL) was similar in efficacy to a liberal transfusion (Hg <9 g/dL) strategy in the critically ill42. However, it remained uncertain whether this observation also applied to patients who had septic shock. This was especially confusing because the EGDT RCT of Rivers and colleagues34 used a transfusion threshold of Hg <10 g/dL, much higher than the conservative TRICC threshold (Hg <7 g/dL). Fortunately, Holst and colleagues43 performed an RCT and again found no difference in mortality between conservative (43% at 90 days) and liberal transfusion thresholds (45% at 90 days, P=0.44, Table 2), but this time in septic shock. Furthermore, there were no differences between groups in ischemic events, adverse events, or numbers of patients who needed life support. Also, when aligned with the results of ProCESS and ARISE described above, it is very reasonable to use a transfusion threshold of 7 g/dL in septic shock. Thus, a conservative transfusion threshold is safe and effective in patients who have septic shock.
The age of transfused blood may alter the outcome in that older stored blood was associated with poorer outcomes in non-controlled or non-randomized studies. In a related RCT, Lacroix and colleagues44 sought to determine whether age of transfused erythrocytes (RBCs) alters outcomes in the critically ill, many of whom had sepsis; patients received 1 unit of either fresh RBCs (stored for 6.1±4.9 days) or standard-issue RBCs (stored for 22.0±8.4 days) (Table 1 and Table 2). There was no difference in 90-day mortality rates between groups (37% vs. 35.3% respectively, P=0.38). All of the secondary outcomes and subgroup analyses aligned with the major finding: no difference between groups in outcomes. Thus, standard issue RBCs are as safe and as effective in the critically ill, and presumably in sepsis.
A large RCT of two strategies45 found no difference in 30-day mortality between early enteral (34%) and parenteral (33%) nutrition in the critically ill. Parenteral feeding was associated with less hypoglycemia and vomiting, but there were no differences in any other secondary outcomes (e.g., infectious complications, 90-day mortality).
A recent randomized controlled trial showed that 20% albumin plus crystalloids did not change mortality compared to patients who received crystalloids alone in patients who had severe sepsis40. The albumin plus crystalloids group had 20% albumin given daily to maintain serum albumin concentration above 30 g/L. Although the albumin group had higher mean arterial pressure and lower fluid balance over the first 7 days, 28-day mortality rates were very similar (31.8% albumin, 32% crystalloid group).
Mortality of sepsis may be decreasing, and so having more survivors of sepsis convinces clinicians and investigators that it’s critically important to understand epidemiology, pathogenesis, genetics, prevention and treatment of long-term complications of sepsis. Many negative RCTs were reported in 2014 that inform the use of EGDT, target mean arterial pressure, albumin infusion, RBC transfusion, nutrition, and the use of statins in severe sepsis and septic shock. Perhaps three changes could improve the possibility of positive RCTs in this field: the use of biomarkers, response adaptive trial design, and a primary outcome of composite organ dysfunction. PCSK9 is an exciting candidate for treatment because anti-PCSK9 strategies target the organism(s), not the host response (a strategy that has almost uniformly failed). Fluid therapies may not require full-on EGDT, but rather emphasize the importance of early recognition and resuscitation of sepsis. Therapeutic use of albumin may be beneficial in septic shock, but requires further evaluation in RCTs. The transfusion threshold in septic shock is now hemoglobin of 70 g/L. A higher MAP target may be beneficial in patients who have pre-existing hypertension because higher MAP may decrease the incidence of AKI and the need for RRT. Nutrition practice can continue with enteral nutrition started on day 2–3 (i.e., early, but there is no indication for very early parenteral nutrition). Early statins at appropriate doses and plasma levels deserve further investigation in trials in sepsis.
Drs. James Russell and Keith Walley report patents owned by the University of British Columbia (UBC) that are related to proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor(s) and sepsis and related to the use of vasopressin in septic shock. Drs. Russell and Walley are inventors on these patents. Drs. Russell and Walley are founders, Directors and shareholders in Cyon Therapeutics Inc. (developing a sepsis therapy). Dr. Russell has share options in Leading Biosciences Inc.
Dr. Russell reports receiving consulting fees from Cubist Pharmaceuticals, formerly Trius Pharmaceuticals (developing antibiotics), Ferring Pharmaceuticals (manufactures vasopressin and is developing selepressin), Grifols (sells albumin), MedImmune (regarding sepsis), Leading Biosciences (developing a sepsis therapeutic), La Jolla Pharmaceuticals (developing a sepsis therapeutic), and Sirius Genomics Inc. (now closed; had done pharmacogenomics research in sepsis).
Dr. Russell reports having received grant support from Sirius Genomics, Ferring Pharmaceuticals, and Astra Zeneca that is provided to and administered by UBC.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 1 28 May 15 |
read | read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)