Keywords
cementless total hip arthroplasty, proximal femur morphology, femoral stem, body mass index
cementless total hip arthroplasty, proximal femur morphology, femoral stem, body mass index
Cementless total hip arthroplasty (cTHA) is one of the most common orthopaedic procedures performed today. Much of its success is due to modern technological advances in implant design that permit cementless press-fit femoral component fixation1–18. Studies have shown that appropriate cementless femoral component size is critical for optimal implant initial stability, and maximizing femoral implant medullary canal fill enhances the endosteal contact between implant and bone to promote bone ingrowth, and therefore improved long-term outcome19–21.
Standard tapered cTHA stems typically rely on three-point fixation, which achieves more proximal load transfer and thereby decreases risk for stress shielding. Nonetheless, subsidence may occur owing to inadequate distal press-fit. More recent designs of anatomic stems aim to reproduce the normal contours of the intramedullary cavity to allow a more natural load distribution over the proximal femur without relying on a specific fixation point22. The clinical relevance of these evolving designs is the inhibition of aseptic loosening and stress shielding, and the optimization of implant longevity.
Because the quality of cementless femoral component press-fit is dependent upon matching the implant size to the dimensions and geometry of the proximal femur, determining the relationship between these parameters and basic patient characteristics such as age, race, gender, and body habitus may assist with operative planning. However, the priority of selecting the largest possible femoral stem size to achieve a tight fit often results in a substantial alteration of the implant’s biomechanical properties. The bending stiffness of a cylindrical stem increases exponentially to the fourth power with increasing a stem diameter, and this phenomenon could be a specific concern in cTHA patients with a proximal femur size and geometry inversely related to their body mass, and when proximal femur size and geometry are incommensurate with body habitus, cTHA patients are potentially subjected to femoral implants that are too stiff or too flexible.
While several investigators have reported significant proximal femur anatomic variations in regard to patient gender, a correlation of body habitus with proximal femur size and geometry has not been reported. The objective of this study was to determine the variability of proximal femur size and geometry in primary cTHA patients, and determine its correlation with patient age, gender, ethnicity, and body habitus.
One hundred twenty-seven patients with primary cTHAs performed at a single institution (University of Texas Medical Branch, Galveston, TX) from 2004 to 2008 were studied retrospectively. Inclusion criteria mandated that the patient was an adult who underwent elective unilateral total hip arthroplasty with a cementless femoral stem and had adequate postoperative radiographs of the involved hip and proximal femur. Exclusion criteria were patients with THAs that were bilateral, revisions, post-infection, acquired (post-traumatic) or due to congenital deformity of the proximal femur, or cemented THA. The study was conducted in compliance with the University of Texas Medical Branch policies and regulations regarding human subject research following study protocol review and approval by the Institutional Review Board (approval IRB #08-156).
Age, gender, ethnicity, and body mass index (BMI) at the time of surgery were documented for each cTHA patient. BMI was defined as the ratio of the patient’s weight (kilograms) to the square of the height (meters). Patients were sub-categorized in accordance with their BMI as follows: underweight (BMI less than 18.5), normal weight (BMI between 18.5 and 24.9), overweight (BMI between 25 and 29.9), obese (BMI greater than 30).
The medullary canal size and cortex thickness of the proximal femur were determined by a series of measurements of the involved hip and proximal femur measured on a postoperative anteroposterior (AP) plain radiograph completed within two weeks of the surgery. The hip/proximal femur radiograph was performed using a standard technique with the x-ray beam centered on the hip with neutral pelvic and lower extremity rotation. All study radiographs were accessed using the digital Picture Archiving and Communication System (IntelliSpace PACS; Philips, Andover, MA, USA) at our institution.
Proximal femur radiographic measurements included the intertrochanteric distance, the inner and outer femur cortical diameters of the femur at the subtrochanteric and diaphyseal regions, as well as the subtrochanteric and diaphyseal cortical thickness. The intertrochanteric distance (ITD) was measured from the tip of the greater trochanter to the tip of the lesser trochanter (Figure 1). The subtrochanteric inner cortical diameter (STID) and outer cortical diameter (STOD) were measured from just proximal to the distal inferior edge of the lesser trochanter perpendicular to the long axis of the diaphysis. The subtrochanteric cortical thickness (STCT) was calculated by subtracting the inner cortical diameter from the outer cortical diameter, and then dividing that figure in half (which assumes that the femur cross-section at that level is circular). The diaphyseal inner cortical diameter (DID), diaphyseal outer cortical diameter (DOD), and diaphyseal cortical thickness (DCT) were determined at a level of 4 cm proximal to the tip of the cementless femoral stem by using the same calculations used for the subtrochanteric region.
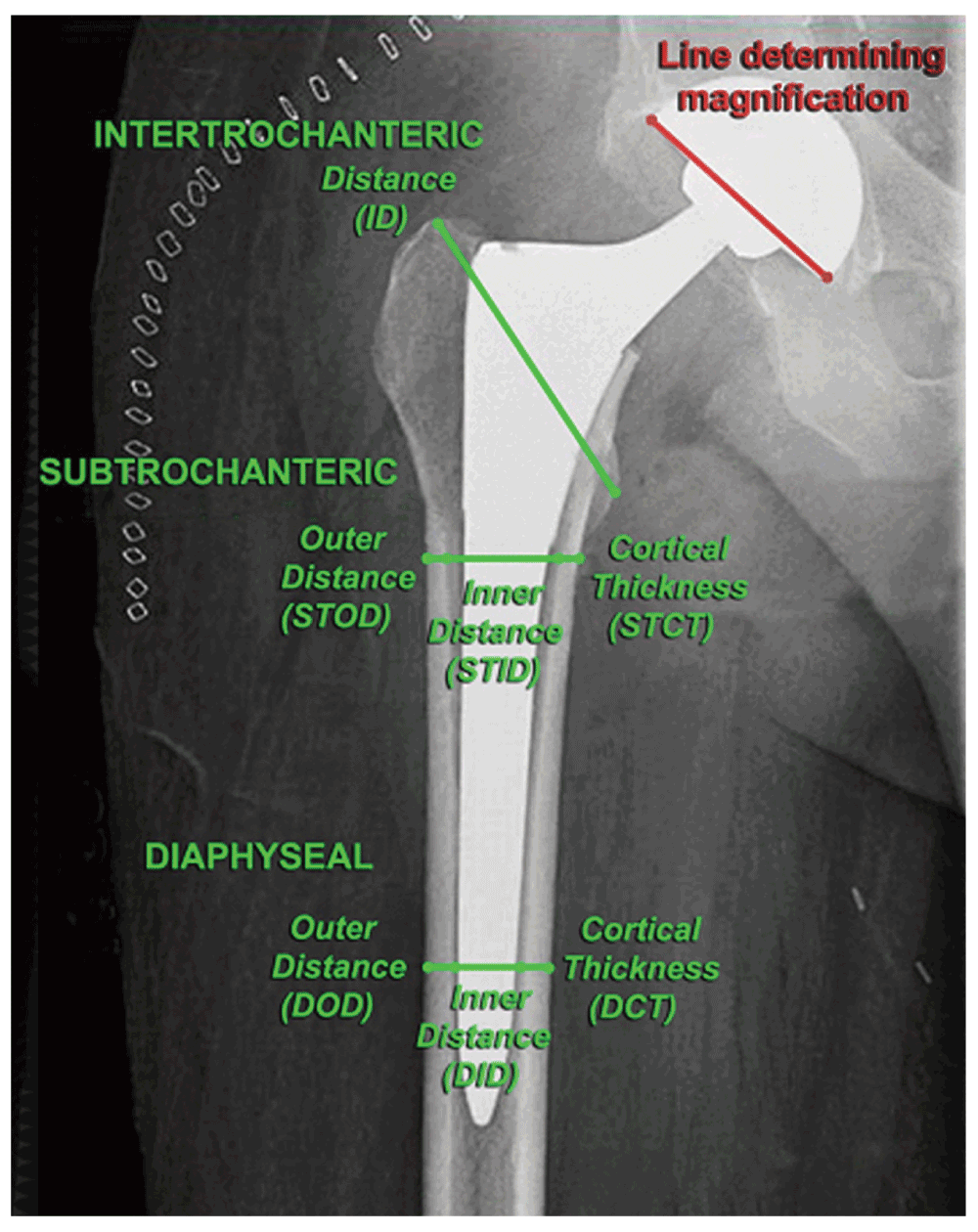
Proximal femur size was established from measurements of intertrochanteric distance (ITD), outer and inner distance, and cortical thickness in the subtrochanteric region (STOD, STID, STCT, respectively), and the same in the diaphyseal region (DOD, DID, DCT, respectively) on AP plain radiographs. The intertrochanteric distance was measured between the tips of the greater and lesser trochanters; the subtrochanteric measurements were taken at the level immediately below the lesser trochanter, and the diaphyseal measurements were taken at the level of 4 cm above the tip of the cementless femoral stem. A magnification factor was established for each radiograph by measuring the outer diameter of the acetabular cup and comparing it to the known outer diameter of the implant obtained from the operative report, and all measurements were adjusted accordingly.
In order for these measurements to reflect the true dimensions of the osseous anatomy, a magnification factor was established for each radiograph. The magnification factor was determined by digitally measuring the outer diameter of the acetabular cup and comparing that measurement to the known outer diameter of the implant obtained from the operative report. This magnification calculation was used to adjust all radiographic measurements.
Proximal femur geometry was assessed using the intertrochanteric-to-subtrochanteric and subtrochanteric-to-diaphyseal ratios suggested in previous reports23,24 as a better measure of proximal femur morphology compared with individual anatomic dimensions. Also, mean total, cortical and medullary cross-sectional areas of subtrochanteric and diaphyseal regions were calculated and compared with patient gender and BMI.
Descriptive statistics (SAS 9.3; SAS Institute Inc, Cary, NC, USA) were used to describe the sample characteristics and distribution of the proximal femur size and geometry (outcome variables). Pearson correlation test and ANOVA were used to test the bivariate association between outcome variable and sample characteristics (age, sex, race, and BMI) depending on the whether the sample characteristics variable was continuous or categorical. Multiple regression models were used to test the association between BMI and outcome variables, adjusting for age, sex, and race. The unadjusted and adjusted means of the outcome variables were also generated using regression models. A p-value threshold of less than 0.05 was considered statistically significant. Post hoc power analysis confirmed that the study sample size has sufficient power to detect a 20% difference in the designated parameters of the proximal femur morphology.
The study consisted of 96 cTHA patients who met the inclusion/exclusion criteria. The patients had the following demographic characteristics: mean age of 60.1 years ranging between 22 and 91 years, 34 patients were females (35.4%) and 62 males (64.6%); 72 patients were Caucasian (75%), 18 Black (18.7%), and 6 Hispanic (6.3%) (Table 1). The BMI data included four (4.2%) patients who were underweight (BMI<18.5); 13 (13.5%) normal (BMI 18.5–24.9); 34 (35.4%) overweight (BMI 25–29.9), and 45 (46.9%) obese (BMI>30) (Figure 2). Proximal femur mean regional dimensions and standard deviations of the outcome variables for all patients in the study are depicted in Table 2.
| Patients | Mean (SD) or N (%)$ |
|---|---|
| Age | 60.1 (14.1) |
| Gender | |
| Female | 34 (35.4) |
| Male | 62 (64.6) |
| Ethnicity | |
| Caucasian | 72 (75.0) |
| Black | 18 (18.7) |
| Hispanic | 6 (6.3) |
| BMI | 30.6 (6.5) |
| BMI Groups | |
| Underweight | 4 (4.2) |
| Normal | 13 (13.5) |
| Overweight | 34 (35.4) |
| Obese | 45 (46.9) |
| Mild | 26 (27.1) |
| Moderate | 11 (11.3) |
| Severe | 8 (8.4) |

Patients were categorized as underweight (BMI less than 18.5), normal weight (BMI between 18.5 and 24.9), overweight (BMI between 25 and 29.9), obese (BMI greater than 30).
| Measurement | Mean (SD) |
|---|---|
| ITD | 74.4 (6.8) |
| STOD | 33.7 (3.5) |
| STID | 28.1 (3.6) |
| STCT | 5.6 (1.1) |
| DOD | 28.4 (3.4) |
| DID | 7.3 (2.9) |
| DCT | 11.1 (2.9) |
No significant correlation existed for patient age or race and all radiographic measurements (Table 3). Males had statistically significant larger proximal femur intertrochanteric (p<0.0001), subtrochanteric (p<0.008), and diaphyseal diameters (p<0.001) as well as cortical thickness compared to females (p=0.002). BMI was directly proportional to outer diameter and cortical thickness in both subtrochanteric and diaphyseal regions; however, BMI was inversely proportional to inner diameter in these same regions. BMI groups exhibited statistically significant correlation for measurements obtained for the subtrochanteric and diaphyseal, but not for the intertrochanteric region. Age and race were not significantly associated with any of the outcome variables (Table 4).
| Measurement | Mean (SE) | P value | ||||
|---|---|---|---|---|---|---|
| Underweight | Normal | Overweight | Obese | |||
| ITD | Unadjusted | 70.6 (3.4) | 72.7 (1.5) | 75.0 (1.0) | 75.0 (0.9) | 0.41 |
| Adjusted$ | 69.4 (2.9) | 69.8 (1.4) | 72.7 (1.0) | 72.0 (0.9) | 0.22 | |
| STOD | Unadjusted | 30.0 (1.7) | 33.3 (0.8) | 33.3 (0.5) | 34.7 (0.5) | 0.02 |
| Adjusted$ | 29.2 (1.6) | 32.1 (0.8) | 32.2 (0.5) | 33.6 (0.5) | <0.0001 | |
| STID | Unadjusted | 21.1 (1.8) | 22.9 (0.8) | 22.3 (0.5) | 22.9 (0.5) | 0.69 |
| Adjusted$ | 20.5 (1.8) | 22.4 (0.9) | 21.8 (0.6) | 22.3 (0.6) | 0.05 | |
| STCT | Unadjusted | 4.5 (0.5) | 5.2 (0.2) | 5.5 (0.2) | 5.9 (0.1) | 0.008 |
| Adjusted$ | 4.3 (0.5) | 4.9 (0.3) | 5.2 (0.2) | 5.6 (0.2) | <0.0001 | |
| DOD | Unadjusted | 24.5 (1.6) | 27.8 (0.7) | 27.9 (0.5) | 29.5 (0.4) | 0.004 |
| Adjusted$ | 23.7 (1.4) | 26.8 (0.7) | 26.9 (0.5) | 28.5 (0.5) | <0.0001 | |
| DID | Unadjusted | 13.1 (1.5) | 13.1 (0.6) | 12.6 (0.4) | 12.6 (0.4) | 0.92 |
| Adjusted$ | 12.8 (1.5) | 13.1 (0.7) | 12.5 (0.5) | 12.5 (0.5) | 0.10 | |
| DCT | Unadjusted | 5.7 (0.7) | 7.4 (0.3) | 7.6 (0.2) | 8.4 (0.2) | 0.0001 |
| Adjusted$ | 5.4 (0.7) | 6.8 (0.3) | 7.2 (0.2) | 8.0 (0.2) | <0.0001 | |
Increasing BMI was significantly associated with higher STOD, STCT, DOD, and DCT values (P=0.02, 0.008, 0.004, and 0.0001, respectively, in unadjusted tests). These significant associations persist after adjusting for age, sex and race (Table 5). Figure 3–Figure 5 graphically represent the associations between BMI and the outcome variables. Table 6 demonstrates a significant linear trend between BMI and STOD, STCT, DOD and DCT. No significant correlation was detected between BMI and intertrochanteric distance, subtrochanteric inner diameter, or diaphyseal inner diameter.
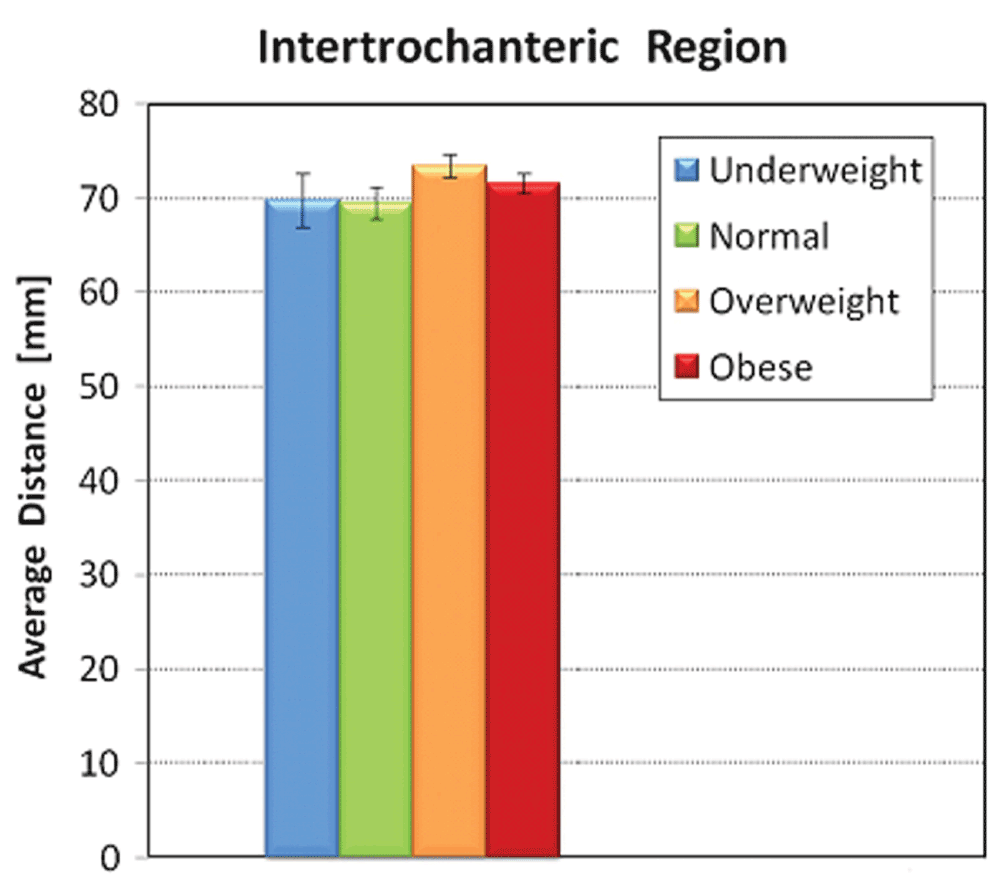
Error bars represent standard deviation.
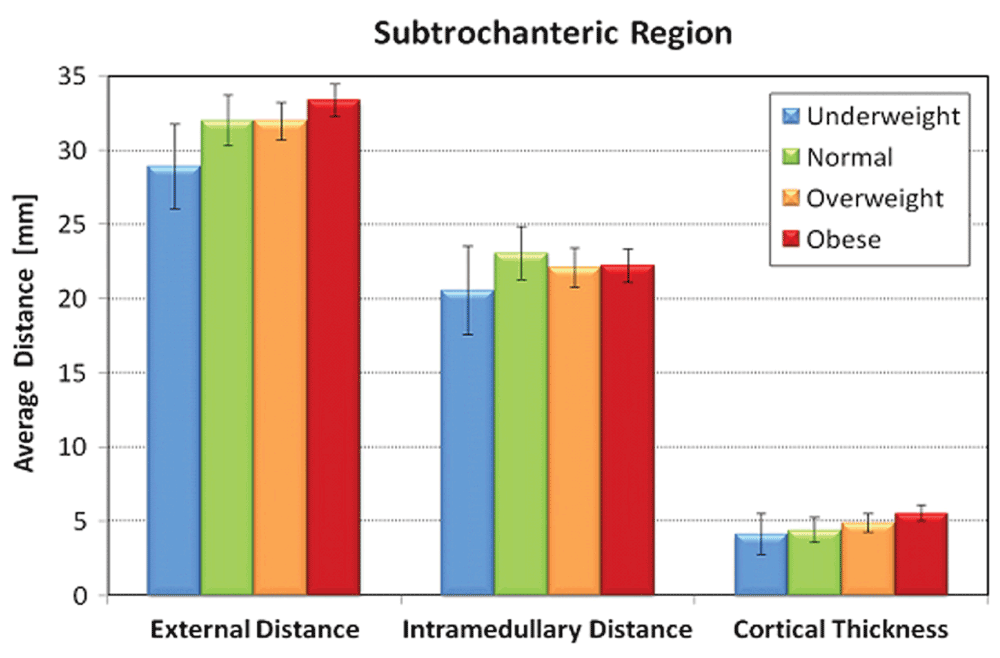
Error bars represent standard deviation.
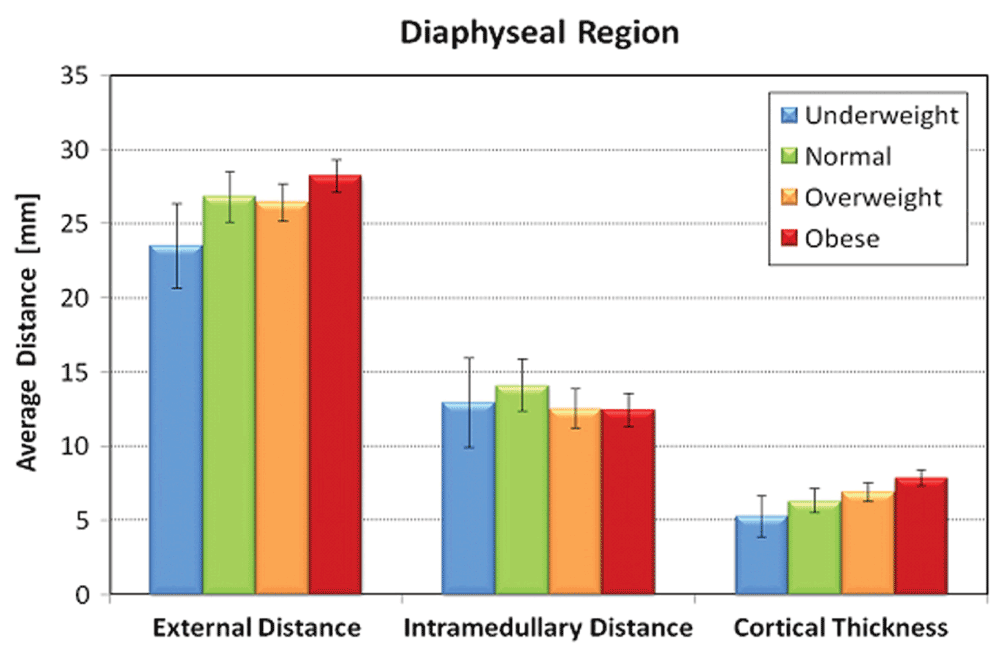
Error bars represent standard deviation.
| ITD/STID | STID/DID | |
|---|---|---|
| Female | 3.3 (0.6) | 1.9 (0.4) |
| Male | 3.4 (0.6) | 1.8 (0.3) |
| (P value) | (0.35) | (0.14) |
The proximal femur geometry assessed form determining the intertrochanteric-to-subtrochanteric and subtrochanteric-to-diaphyseal did not demonstrate a statistically significant association with patient gender (Table 6). The mean cross-sectional areas of subtrochanteric and diaphyseal regions versus gender and BMI group are depicted in Table 7 and Table 8, respectively. No statistical significant associations were observed between these parameters of the proximal femur geometry and patient gender. The normal-weight group had the largest average canal width and on average received the largest femoral stems, whereas the overweight and obese patients had smaller canals, and received smaller femoral components (Table 8).
| Subtrochanteric Region | Diaphyseal Region | ||||||
|---|---|---|---|---|---|---|---|
| Total Area (cm2) | Cortical Area (cm2) | Medullary Area (cm2) | Total Area (cm2) | Cortical Area (cm2) | Medullary Area (cm2) | ||
| Female | 7.9 | 4.2 (54%$) | 3.7 (46%$) | 5.3 | 4.2 (79%$) | 1.1 (21%$) | |
| Male | 9.6 | 5.3 (56%$) | 4.3 (44%$) | 7.0 | 5.6 (80%$) | 1.4 (20%$) | |
| P value | 0.25 | 0.62 | |||||
| Proximal area | Distal area | |
|---|---|---|
| BMI group | ||
| Underweight | 441 | 85 |
| Normal | 588 | 147 |
| Overweight | 518 | 114 |
| Obese | 543 | 128 |
Several cTHA studies have emphasized the importance of femoral fit in achieving primary implant stability, reducing early prosthetic loosening, and encouraging bony ingrowth2–9. Understanding the bony anatomy of the proximal femur is a prerequisite for optimizing femoral implant press-fit and determining the impact of factors such as ethnicity, gender, age, and body habitus10,12. At present, clinical priority is given to selecting the largest possible femoral stem to accomplish a tight fit, despite increased implant size association with a considerable change in the stem biomechanical properties (four-power increase in bending stiffness in relation to stem diameter). Hence, the size and geometry of the proximal femur may have significant implications for the cementless femoral stem biomechanical characteristics, especially in patients for whom a large discrepancy exists between their proximal femur dimensions and their body habitus.
Previous anthropometric studies have compared the variability of femoral bony architecture among different races. Travison et al.25 used bone densitometry to show that African-Americans have larger intertrochanteric and diaphyseal outer diameters compared to Hispanics and Caucasians. Marshall et al.26 determined that African-Americans and Asian men have greater femoral neck and shaft mean cortical thickness compared to Caucasian men. Other studies27,28 demonstrated that African-American women had a larger cross-sectional area, larger outer diameter, smaller inner diameter, and thicker cortices in the femoral neck and shaft compared to Caucasian women, and also have smaller inner and outer diameters, but thicker cortices in the intertrochanteric region. This is in contrast with the results of the present study, which did not establish a statistically significant correlation between race and the proximal dimensions. These findings corroborate those of Peacock et al.29 and Saeed et al.30, who reported computed tomography measurements of the proximal femur to be independent of race for both sexes. These studies, however, have substantiated significant differences in bone mineral density among these ethnic groups25,26,29,31.
The literature has shown that men generally have a larger bony architecture compared to women, even when adjusted for confounding variables29,32,33. These differences have promoted the development of gender-specific arthroplasty implant designs. Kostamo et al.34 in a large primary THA cohort demonstrated that differences in clinical outcome scores were found only in the WOMAC pain score in favor of the female cohort (39.4 versus 36.1), whereas the survivorship and revision rate were not significantly different. Men required larger femoral stems with greater stem lengths, neck offset, and neck lengths. Despite the gender variations in proximal femur anatomy, the authors concluded that current implant systems in their versatility sufficiently address the different size and offset needs of both male and female patients. Similar results have been reported for gender-specific total knee arthroplasty components35.
In the present study a statistically significant difference in BMI was determined between males and females for all outcome variables (Table 4). The proximal femur morphology assessment involving ratios of geometric measures as opposed to individual anatomic dimensions demonstrated that, although the single measures of the specific proximal femur regions exhibited statistically significant differences (Table 2), the intertrochanteric-to-subtrochanteric and subtrochanteric-to-diaphyseal ratios did not (Table 6 and Table 7). These findings suggest that although the bony anatomy of men is larger than that of women, the proportional increase in proximal femur size, and subsequent shape, is similar for both. Also, the cortical and medullary cross-sectional areas as a percentage of the total cross-sectional area in the subtrochanteric and diaphyseal regions were similar for both men and women (Table 8). Lamellar bone stiffness is largely determined by its radius and cortical content. Therefore, although women may exhibit less lamellar bone stiffness, this difference is in proportion to that of men. Furthermore, a disparity did not exist between anatomic regions for either sex, and this may suggest that although men may require larger femoral implants compared to women, gender-specific implant geometry may not be necessary as the proximal femur regional anatomic proportions are equivalent.
Bone mass decreases with age after its peak in the third decade of life; however, studies have shown that the outer diameter of bone slightly increases with age, possibly owing to continued periosteal appositional growth25,33,36,37. But to produce an age-associated decrease in bone density while its outer diameter is increasing would require thinning of the cortex. Our study did not demonstrate a statistically significant correlation between age and proximal femur geometry, although the aforementioned trends may be seen (Figure 6 and Figure 7). Bone adaptation to mechanical loading (Wolff’s law) is site specific because of the unique magnitude, direction, and type of load in each anatomic region. Among the major determinants of the loads experienced at the hip joint and subtrochanteric region of the femur is body weight. In a bone densitometry study, Petit et al.38,39 demonstrated that the femoral neck and shaft in children and adolescents who were overweight due to a higher percentage of lean body mass had larger diameters, cross-sectional areas, and cortical thicknesses compared to normal-weight individuals. Van der Meulen et al.40 indicated that of age, pubertal stage, body mass, and height, body mass is a stronger predictor of femoral cross-sectional properties than age, pubertal stage, or height. Moreover, these authors concluded that the correlation of body mass with femoral cross-sectional structure is independent of gender. Interestingly, other authors have indicated that body weight and femoral dimensions may possess common genetic factors41–43.
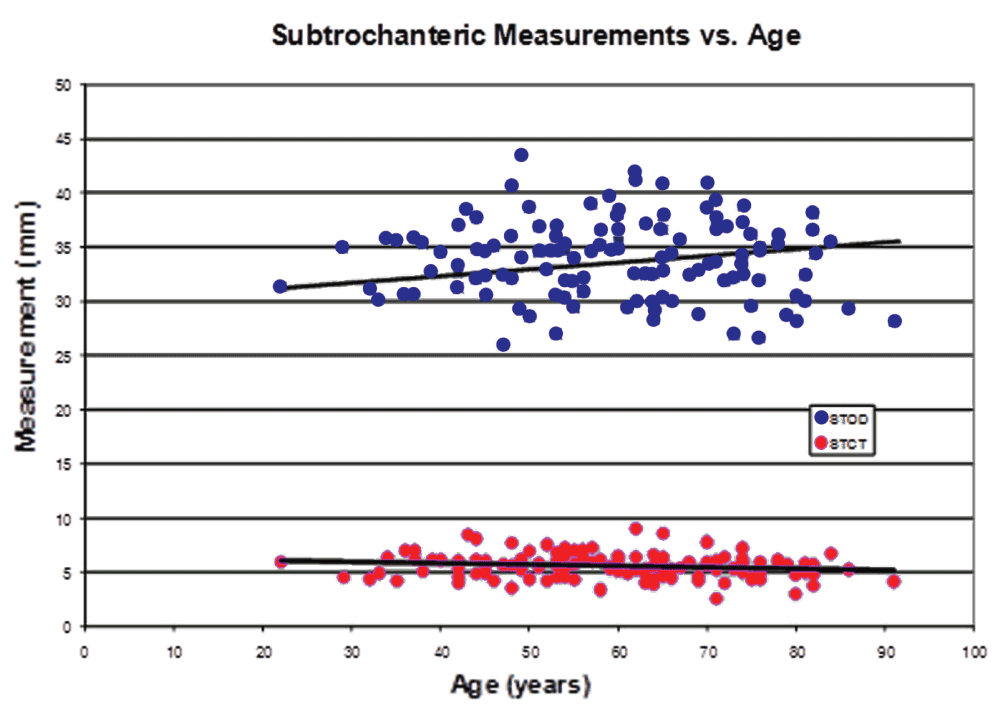
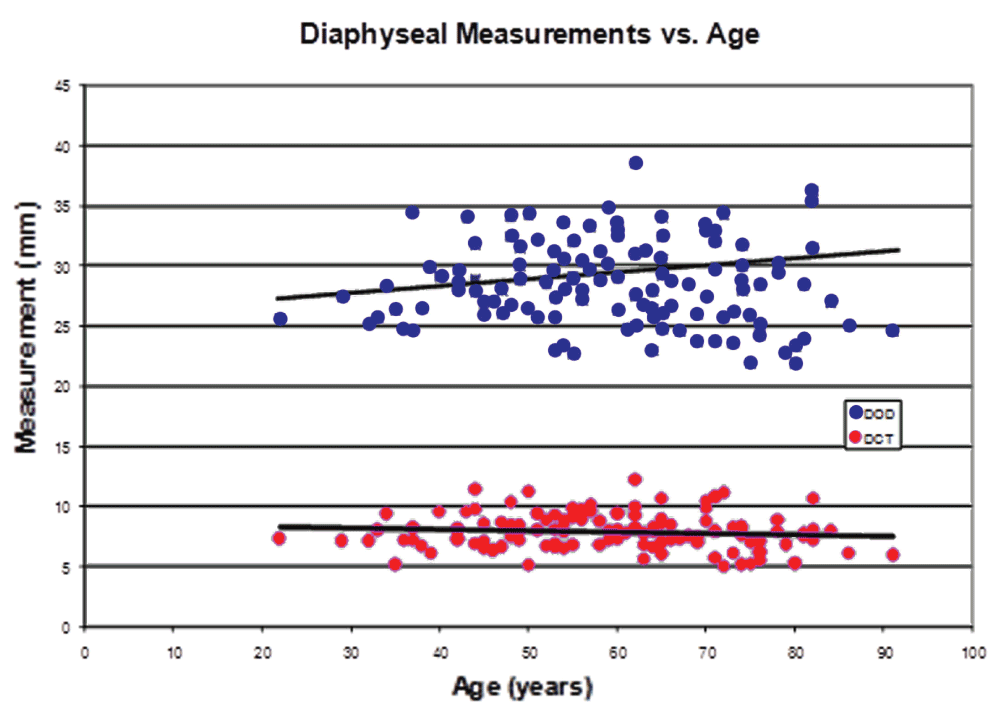
In our study, there were significant differences in subtrochanteric (STOD, STCT) and diaphyseal (DOD and DCT) measurements versus individual BMI groups (Table 4), and versus BMI as a continuous variable (Table 5). This correlation demonstrated the significant linear trends between STOD, STCT, DOD and DCT and BMI. The subtrochanteric inner diameter (STID) versus BMI groups demonstrated statistical significance only after being adjusted for age, sex, and race; the same was observed for the diaphyseal inner diameter region (Table 5). In both regions, the outer diameters increased with larger BMI, but the cortical thickness also increased, resulting in fairly consistent inner diameters among BMI groups. On the other hand, the differences in intertrochanteric measurement (ITD) were not statistically significant. Intertrochanteric distance can be highly variable on anterior-posterior plain radiography with varying degrees of hip rotation. Although AP hip radiographs in our institution are routinely attempted with the femur in neutral alignment, many patients with longstanding hip disease may have had contractures causing external proximal femur rotation. Our study showed a less than 5% variation in the ITD measurements.
One concern with press-fit femoral components is the disparity that frequently exists between the anatomy/configuration of the human proximal femur and the specific design and size limitations of any implant system. Our study confirms that a wide variation exists in proximal femur geometry among hip arthroplasty patients. The notion that current off-the-shelf femoral components do not accurately accommodate this wide variation is supported by reports of persistent bone stress shielding or implant subsidence despite advances in stem design. All cTHA study patients received a tapered stem, and the average proximal and distal cross-sectional area of the implanted femoral component according to BMI group is depicted in Table 8. The normal-weight group had the largest average canal width and, on average, received the largest femoral stems. By comparison, overweight and obese patients had smaller canals, and received smaller femoral components. These findings suggest that the normal-weight patients may receive implants which for their body habitus, are excessively stiff; conversely, obese patients may actually be receiving implants that are not stiff enough. Although, to our knowledge, there has not been a study that relates implant stiffness and fatigue resistance to implant longevity among different BMI groups, several studies suggest no differences in the clinical or radiographic outcomes among different BMI groups44,45.
A correlation of body weight with the incidence or severity of thigh pain has not been demonstrated. Several studies have also attempted to correlate thigh pain with stem size; however, their results have been inconclusive7,46. Stem size and/or geometry clearly influences thigh pain, as a stem with a better fit will result in more stability and bony ingrowth, and theoretically, less thigh pain47,48. Similarly, stem stiffness may influence thigh pain as a stem with a stiffness closer to that of the host femur would result in more uniform stress transfer48,49. Body weight could also have a great influence on the incidence of thigh pain, especially if there is a considerable mismatch of host bone to implant stiffness. In the present study, normal-weight patients had smaller outer diameters with thinner cortices compared to obese patients, but actually received larger implants owing to their larger canals. Theoretically, this mismatch in stiffness may the cause of thigh pain in normal-weight individuals, however, this has not been proven clinically44.
The present study has several limitations. Selection bias may have been introduced into the study - the typical study patients were older and obese, while younger, underweight or normal-weight patients were underrepresented. The study demographic data may also be inconsistent or incomplete regarding ethnicity and body mass. The designation of ethnicity was used as recorded in the medical records without a standard definition or verification. Also, the study design did not account for physical activity, or the lack thereof, as well as any differences between low and high body mass as previously discussed. In addition, each patient underwent cTHA with a press-fit femoral component, and the amount of endosteal cortical bone removed through reaming and/or broaching was undetermined. Finally, the complexity of the proximal femur geometry was assessed only with plain radiography.
In summary, the present study shows a strong correlation between the proximal femur dimension and gender, but not with age or ethnicity. This correlation is statistically significant for outer diameter and cortical thickness in both the subtrochanteric and diaphyseal regions of the proximal femur. Conversely, there is a non statistically significant reverse trend between BMI and inner medullary diameter in both regions. The study findings suggest that gender-specific femoral implants based on presumed differences between male and female proximal femur size but not geometry may be warranted. The development of cTHA femoral components that more accurately address the proximal femur variations specifically reflected by the patient overall and local biomechanical demands (BMI, bone quality, etc.) may have considerable merit in extending implant longevity.
The present study demonstrates a strong correlation between gender and the size of the proximal femur, but no correlation with age or race. There is a direct correlation between BMI and proximal femur size; this correlation is statistically significant for outer diameter and cortical thickness in both the subtrochanteric and diaphyseal regions. Conversely, there is a non statistically significant reverse trend between BMI and inner medullary diameter in both regions.
Study results suggest that gender-specific cTHA femoral component matching male versus female proximal femur size may be indicated, and the development of femoral stems that addresses the proximal femur anatomic variations together with its matched biomechanical properties may have considerable merit.
F1000Research: Dataset 1. Raw data for ‘Proximal femur size and geometry in cementless total hip arthroplasty patients’, 10.5256/f1000research.6554.d4979650
DLM - data collection, data analysis, manuscript preparation; RWL - study design, manuscript preparation; ZG - study design, data analysis, manuscript preparation. All authors have seen and agreed to the final content of the manuscript.
We thank Alai Tan, PhD, for assistance in statistical analysis and Suzanne Simpson, BA, for editing the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 23 Jun 15 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)