Keywords
Serum concentration, IgG antibodies, PCV-10
Serum concentration, IgG antibodies, PCV-10
Streptococcus pneumoniae (pneumococcus) is still the number one cause of morbidity, among infants and the elderly globally1. It is estimated that 150.7 million cases of pneumonia occur annually among infants2. Out of these, over 30% are usually severe enough to require admission to a hospital. Pneumonia accounts for >4 million deaths annually in under-developed countries3. It also contributes 20% of the total child deaths in Kenya annually4. Although there are antibiotic interventions, deaths caused by Pneumococcal Disease (PD) still remain high.
According to the World Health Organization (WHO) over 70% of child deaths in 2008 were as a result of pneumococcal disease complications5. In developing countries, pneumococcal septicemia and meningitis account for 20% and 50% of severe PD cases, respectively6. The surge in anti-biotic resistant Streptococcus pneumoniae serotypes is an increasing global concern posing serious treatment challenges7. Because of the relatively high level of success of pneumococcal conjugate vaccines, the WHO has recommended that vaccines with broader serotype coverage be developed8.
Accessibility to a safe and universally relevant sero-type inclusive vaccine is the surest way of curbing morbidity and mortality due to pneumococcal disease9. Although the WHO has recommended inclusion of PCVs in various national infant immunization programs10, it has not been very effective for >90% of under-developed countries. This is largely due to missing Streptococcus pneumoniae serotypes known to be circulating in developing countries11. PCV-10 contains serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F and 1, 5 and 7F12. Pneumococcal infections, irrespective of serotype, can be successfully treated with antibiotics, but the increasing resistance among pneumococci to antibiotics has highlighted the need for prevention, which can be achieved by vaccination13. Both 23-valent pneumococcal polysaccharide vaccine (PPV) and 10-valent pneumococcal conjugate vaccines are currently being used in Kenya14 yet no study has been done to confirm whether they are immunogenic against PD or not. PPV and PCV-10 were not formulated on the basis of exclusive serotypes found in Kenya and also despite their continued use, pneumonia remains the most killer of children in Kenya15. This has excited an interest in development of new type of vaccines that include all serotypes circulating in Kenya.
Considering the antibiotic resistance levels, prevention of pneumococcal disease early in life is necessary and thus so is the need for maternal and early infant immunization. According to the WHO, the effectiveness of pneumococcal conjugate vaccines can be evaluated on the basis of serum immunoglobulin G (IgG) levels17. A worldwide threshold level of 0.35 mg/dl after three doses at 6 weeks, 10 weeks, 14 weeks and in some cases a booster dose after 12 months has been recommended by WHO as a reference value for assessing immunogenicity of pneumococcal conjugate vaccines18.
On 14th February 2011, Kenya rolled-out the PCV-10 which is the vaccine that includes >50% of the serotypes circulating in Kenya19. Since the launch of the vaccine in February 2011, there are a rising number of children enrolling, yet it is not clear to what extent the vaccine is protective. Studies have not been conducted on the effectiveness of the pneumococcal vaccine in Kenyan children. Therefore there is still a need to evaluate the immunogenicity to determine the effectiveness of the vaccine in Kenyan children20.
S. pneumoniae has over 90 serotypes, eight of which are contained in the PCV-10 (Synflorix) currently included in the Kenya Expanded Program on Immunization (KEPI). Pneumococcus serotypes vary a lot and their epidemiology is based on age, time period and geographical area21. Currently, there is no information on the pneumococcal serotypes present in Nairobi and other pneumococcal disease endemic areas in Kenya. It is possible that they are different from those included in the vaccine, putting the effectiveness of the vaccine in question. Over 70% of global annual deaths caused by PD is among children from developing countries22. Since the launch for free public use of PCV-10 in February 2011, there is an increase in the number of children being immunized yet its safety and immunogenicity has not been established. No data have been published on the immunogenicity after each dose in a series, or on the relative immunogenicity in Kenyan children of different ages. We therefore evaluated the concentration of PD immune antibodies as indicators of immunogenicity.
A cross-sectional study to evaluate PCV-10 immunogenicity among Kenyan infants was conducted between April 2013 and September 2013 among infants aged 1–12 months attending the Mbagathi District Hospital Child Welfare Care Clinic (Nairobi, Kenya). This study was funded by the National Council for Science, Technology and Innovation (NACOSTI) and ethical approval was obtained from the Kenyatta University Ethics Review Committee (ERC).
The majority of the patients treated at the hospital came from the neighbouring Kibera slum – an informal settlement. Most of these children presented with PD complications.
To determine the minimum sample size, the following formula was used: Sample Size (n) = 38423
A minimum of 384 infants from among those who received the vaccine were required for the study with a precision level of 5%. is the assumed prevalence of pneumococcal disease among vaccinated childrenm, is the desired margin of error around the estimated prevalence, herein taken to be 5% and for 95% confidence z=1.96.
The subjects were enrolled by simple consecutive and convenient sampling. This entailed determination of the sample size as above and the enrolment of subjects as they were admitted to the clinic for administration of Vitamin A supplements. A total of 318 infants were recruited on a ‘first come, first sampled’ basis and this represented a response rate of 83%24. An equivalent number of mothers were enrolled along with their infants to investigate herd immunity.
Infants who attended the Child Welfare Clinic of Mbagathi District Hospital for vaccination and who had completed 3-doses of PCV-10 at least 4 weeks earlier were recruited. Participating infants were aged 12 months and below. Study infants’ biological mothers were approached at the clinic for informed consent. A total of 318 biological mothers to infants who were eligible for this study were also recruited to investigate if the vaccine confers herd immunity.
Infants with a medical condition (e.g. one that requires frequent visits to hospital) which would interfere with the assessment of the study objectives were excluded from the study. All subjects not meeting the general inclusion criteria or whose mothers refused to sign the consent form were excluded.
All biological mothers of the infants involved in the study were approached at the Child Welfare Clinic of Mbagathi District Hospital by the researcher as they brought their children for weight check and vitamin A administration to sign a written informed consent document. This was done after reading the document and receiving a study explanation in Kiswahili as appropriate (Appendix I, translated to English in Appendix II). They also had opportunity to ask questions relevant to the study which facilitated the informed participation in the study.
Structured questionnaires were formulated by the main researcher and moderated by the study supervisors. Piloting was done at the Kiambu District Hospital and corrections made before the main study. Standardized questionnaires were thereafter used to collect quantitative data from the participants’ mothers.
Serum samples were collected by qualified and government registered phlebotomists working at the hospital. Coagulant-free vials were used to store the collected sample25. After child preparation (entailing the presence of the mother to ease tension), a 5ml capillary blood sample (heel stick sampling) was collected aseptically into the vials. Venous blood samples were collected from the mothers. Specimens were stored at room temperature until a clot formed (usually 15–45 minutes), then centrifuged to obtain serum specimens for assay. The serum was analysed within 24 hours then stored at 4°C. Serum samples that needed to be analysed out of this range of time were stored at -20°C. Samples were then transported to the Human Diagnostics World Laboratory on dry ice.
All serum samples were analysed using standard ELISA technique26. A pneumococcal antibody threshold concentration limit of 0.35 mg/dl indicated sero-protection27. Samples with antibody levels below this value were considered as lacking sero-protection.
Responses to the well-structured questionnaire were collected. All the study questionnaires remained in safe custody of the principal investigator to ensure confidentiality. Data on IgG levels were collected through assaying serum samples for children and mothers enrolled. Data were entered, and stored on Statistical Package for Social Scientists version 20 (SPSS) until analysed.
Scientific and ethical approval and authorization to conduct the study was sought from Kenyatta University (KU/R/COMM/51/37-2). Signed informed consent was sought from study participants after a clear explanation of the study and its purpose. The data collected and reported were in a form that did not allow identification of individual participants. Study numbers (Barcodes) and not personal identification was used in this study.
SPSS version 20 was used28. Cross-tabulations and correlation analysis were used to generate relationships between dependent and independent variables, respectively. Ninety-five percent confidence intervals were used. Univariate analysis was done to determine the most significant variable that affects the PCV-10 vaccine immunogenicity.
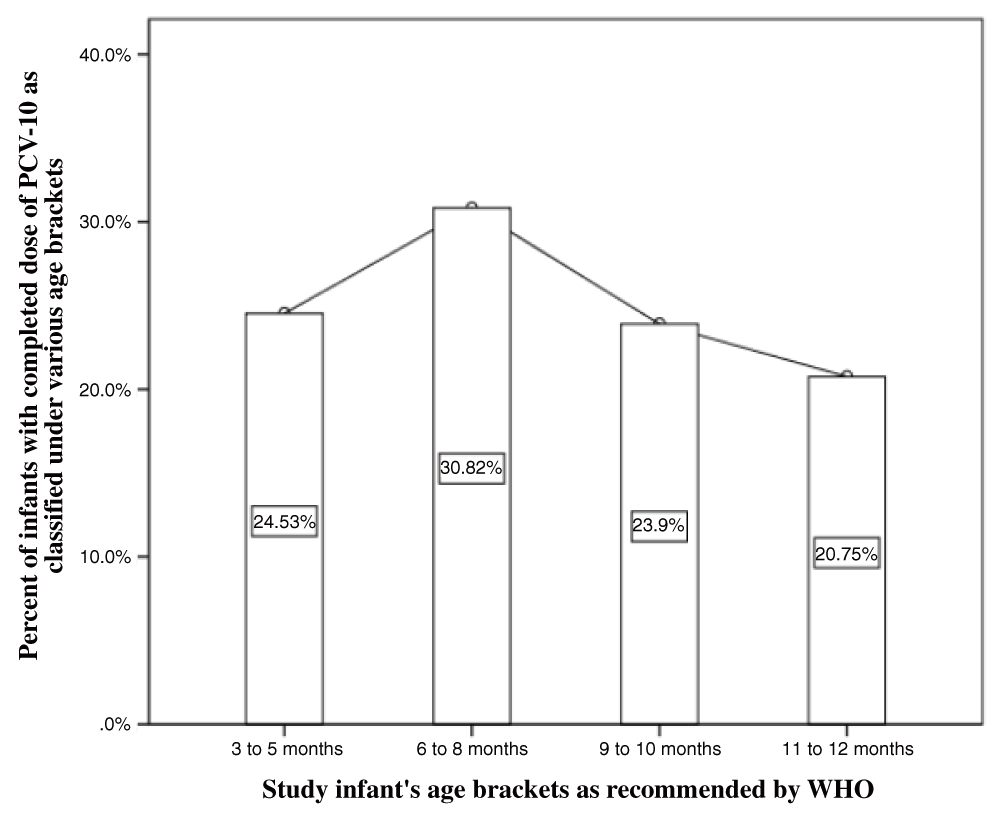
Most of the infants (30.8%) involved in this study were aged between 6–8 months. The second largest group who constituted 24.5% of the study group were aged between 3–5 months (Figure 1).
Our results show that the majority of infants (83%) presented antibody levels between 0.34–0.36 mg/dl after completion of 3-doses of PCV-10 vaccination. 8% of infants presented with 0.37–0.39 mg/dl antibody levels (mg/dl) (Table 1).
This was followed by 4% of infants presenting 0.30- mg/dl to 0.33 mg/dl serum concentration of PD-specific IgG antibodies. The remainder had IgG antibody titres ranging between 0.25 mg/dl and 0.29 mg/dl and ≤0.25 mg/dl respectively.
The results from the majority of the subjects (83%) correlate with the threshold recommended by the WHO29. These data were also consistent with the data obtained from studies on the level of bacterial polysaccharide immune globulin needed to prevent pneumococcal otitis media and IPD30 among Ugandan children residing in Kampala. The findings are relatively higher than those found in the study done among Brazilian infants whose IgG antibody titres between 0.30 mg/dl to 0.36 mg/dl were ≤50% of the total subjects studied31.
The data from our study shows that use of alcoholic drinks, maternal diet during pregnancy and breastfeeding frequency affect serum antibody titres. The IgG antibody titres for subjects who consumed alcoholic drinks during pregnancy were relatively lower as compared to those who did not (Table 2). The results also show that the majority (>68%) of the study subjects had antibody titres between 0.34–0.36 mg/dl (Figure 2) which means that majority of them had developed immunity against PD. This study also found out that there was multi-collinearity between IgG antibody levels (mg/dl) for mothers and several variables. These variables were: consumption of alcoholic drinks by study infants’ biological mother (r =.595, p > 0.01); maternal diet during pregnancy (r =.137, p > 0.05); breast feeding frequency (r =.220, p > 0.01); gap with other children (r =.133, p > 0.05); chronic illness (r =.154, p > 0.01); and IgG antibody levels (mg/dl) for study infant’s biological mothers (r =.675, p > 0.01). Study by CDC shared similar results with this study particularly when comparing IgG antibody levels (mg/dl) for study infant’s biological mothers and IgG antibody levels (mg/dl) for infants. The study found out that there was a relationship between levels of reactive IgG among mothers and IgG antibody levels (mg/dl) for infants. Other studies however, did not find a significant correlation between levels of IgG among mothers and socio-demographic factors.
In addition, most of the infants in this study who were breast-fed more than five times a day and whose maternal diet was balanced during pregnancy had their IgG levels higher or equal to the recommended threshold (Table 3). It should be noted that the total percentage of infants with a serum concentration of PD-specific IgG between 0.34 mg/dl – 0.36 mg/dl was 76.1% in our study, relatively higher than the score of 24% within a six month period in a study done among infants in South Africa32. This is probably because of the Streptococcus strains included in the vaccine formulation. Out of the ten strains included in the vaccine, more than half are found in Kenya whereas these strains may not be found in South Africa33. Socio-demographic patterns may have also played a role in causing the disparity in immunogenicity of the vaccine between the two studies.
The PCV-10 vaccine was found to be immunogenic against PD four weeks after three doses among infants attending the Child Welfare Clinic at Mbagathi District Hospital in Nairobi. Socio-demographic factors which include use of alcoholic drinks by infants’ biological mothers during pregnancy and study infant chronic illness negatively affect the development of PD specific IgG. Balanced maternal diet during pregnancy, breastfeeding frequency (more than three times per day), was shown to have a significant positive effect on serum pneumococcal IgG levels among infants.
F1000Research: Dataset 1. Immunogenicity of 10-valent pneumococcal conjugate vaccine among infants at Mbagathi District hospital, 10.5256/f1000research.6087.d4903535
Written informed consent for publication of clinical details was obtained from all participants and the mothers of the infants enrolled in the study.
Michael Walekhwa was the main researcher, directly involved in the collection and coordination of data and sample collection, analysis and report writing. The study was supervised by Dr. Margaret Muturi of Kenyatta University who was very instrumental in proposal and thesis development. Prof. Elizabeth Bukusi was very helpful with regard to technical aspects of laboratory work.
This study was funded by the National Commission for Science, Technology and Innovation to Michael Walekhwa (NCST/5/003/3rd CALL, MSc/118). The funds entirely facilitated all research work.
I sincerely thank Dr. Margaret Muturi for her professional input that has seen this research reach this level, not to mention her unmatched patience and motherly treatment. Prof. Elizabeth Bukusi’s courtesy, organization and professionalism were incredible. I thank the National Council of Science and Technology (NACOSTI) for the funding. Mbagathi Hospital staff offered me incredible support and advice.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 23 Jun 15 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)