Keywords
Systematic review, meta-analysis, coronary artery disease, coronary angiography, myxoma
Systematic review, meta-analysis, coronary artery disease, coronary angiography, myxoma
Myxomas are the most common primary cardiac tumors, although extremely rare. As an example, in one series of over 12,000 autopsies, only two were identified, for an incidence of less than 0.02 percent1. Histologically, these tumors are composed of scattered cells within a mucopolysaccharide stroma. The cells originate from a multipotent mesenchyme that is capable of endothelial and neural differentiation2. Myxomas produce vascular endothelial growth factor, which probably induces angiogenesis for tumor growth3.
Macroscopically, the tumor surface can be smooth, friable or villous. The tumor diameter varies, ranging from 1 to 15 cm, with a weight typically between 15 and 180 g (mean, 37 g). Friable tumors are more prone to embolization, while larger tumors present with cardiovascular symptoms4.
The mean age of patients with myxomas is 56 years and 64–70% are females. However, myxomas have been described in patients ranging in age from 3 to 84 years. Approximately 86% of all myxomas occur in the left atrium, and most of the remainder is found in the right atrium. Over 90% are solitary4,5.
The cardiovascular manifestations depend upon the anatomic location of the tumor. In a series of 112 consecutive cases of left atrial myxoma: (1) cardiovascular symptoms were present in 67%, more commonly in the form of mitral valve obstruction (mostly cardiac failure or malaise). Cardiac auscultation abnormalities occurred in 64%, essentially pseudo-mitral valve disease in 53.5% and more rarely the suggestive tumor plop in 15%. The most frequent electrocardiographic sign was left atrial hypertrophy in 35%, whereas arrhythmias were uncommon. (2) Embolic symptoms were observed in 29%, essentially cerebral emboli with stroke, with men at greater risk. (3) Constitutional symptoms were observed in 34% with fever, weight loss, or symptoms resembling connective tissue disease4. Right atrial tumors are more commonly associated with signs and symptoms of right heart failure. Tumor fragments can embolize to pulmonary vasculature and cause symptoms consistent with pulmonary emboli, or in the presence of a patent foramen ovale or atrial septal defect, hypoxemia or systemic emboli6,7.
Echocardiography is a widely available, simple and noninvasive approach, which in almost all cases precisely locates the tumor and defines its extent. In addition, transesophageal echocardiography (TEE), cardiac magnetic resonance (MRI) and ultrafast computed tomography (CT) have also proved their usefulness in diagnosis8,9.
Once a presumptive diagnosis of a cardiac myxoma is made, surgical removal is indicated because of the risk of embolization or of sudden cardiac death. The prognosis for patients with solitary myxomas after surgical resection has been excellent with mortality rates of about 4%. Late recurrences are infrequent and reported to occur in 0.4–5% of patients10.
Several studies have attempted to estimate the rate of cardiac myxomas with concomitant CAD and we therefore conducted a systematic review and meta-analysis of observational studies to summarize the point prevalence of CAD in adults with these tumors.
We carried out a systematic review and meta-analysis of prospective and retrospective observational studies following the PRISMA statement (Supplementary Material S1)11. Initially, a search in the main databases (MEDLINE, The Cochrane Library, and LILACS) was performed, searching for studies with similar objectives and methodology. No similar study was found.
A systematic MEDLINE search was performed with the medical subject headings (MeSH) terms (“Myxoma”[MeSH] AND “Coronary Angiography”[MeSH]) OR (“Myxoma”[MeSH] AND “Coronary Disease”[MeSH]), looking for trials in English, Spanish and Portuguese, published until December 2014, that performed coronary angiography in patients with cardiac myxomas. At the same time, a systematic LILACS search was also performed using the same MeSH terms and search strategy.
We designed a relatively strict set of inclusion and exclusion criteria and considered studies meeting these criteria to be of acceptable quality. The study selection criteria were: (1) observational studies, with prospective or retrospective data collection; (2) studies that provided a measure of CAD prevalence in adult patients with cardiac myxomas; (3) studies that included at least five cases of cardiac myxomas; (4) studies in which at least 75% of the adult cardiac myxoma patients had coronary angiographies; (5) angiographic and demographic data systematically reported.
Two researchers, according to the previously established inclusion criteria, then independently reviewed the titles returned by the systematic search. Exclusion by duplicity, title, abstract and full text analyses was independently performed and discrepancies in each stage were solved by consensus after discussion. The selected articles were read in full to confirm eligibility and their data was tabulated and reviewed for the statistical analysis. The second researcher independently double-checked the extraction of primary data from every study.
The meta-analysis of the pooled prevalence data, as well as associated graphic results was performed using the Comprehensive Meta Analysis software, version 2.2.064. Other computations were performed with IBM SPSS Statistics for Macintosh, Version 22.0.
Heterogeneity of accuracy measures was explored with the I2 estimate (inconsistency measure) from Cochran Q according to the formula: I2 = 100% x (Cochran Q – degrees of freedom)/Cochran Q. This describes the percentage of the variability in effect that is due to heterogeneity rather than sampling error (chance)12. Publication bias was graphically assessed using funnel plot, Egger's test and Trim and Fill method13–15.
A flow chart of the studies evaluation is shown in Figure 1. These latter studies were excluded because of a lack of angiographic data in at least 75% of patients with cardiac myxomas in the populations examined. Thus, a total of 6 studies evaluating the prevalence of CAD were selected according to the aforementioned criteria.
For each study, demographic characteristics, the proportion of adult cardiac myxoma patients who underwent coronary angiography and the location of the tumors are listed in Table I. The criteria used to define the presence of CAD and associated treatment when described is listed in Table II. The prevalence rates of clinically confirmed CAD for each of the 6 studies are reported in Figure 2.
| Study | Male/Female | Mean Age (Years ± SD) | Coronary Angiography | Intracardiac Location of Myxomas |
|---|---|---|---|---|
| Van Cleemput et al.16 | 7/18 | 57.37 ± 9.97 (range 38–71) | 19/25 (76%) | LA: 23 RA: 2 |
| Ergünes et al.17 | 8/9 | 54.64 ± 13.02 (range 27–75) | 14/17 (82%) | LA: 14 RA: 3 |
| Gismondi et al.18 | NP | NP | 18/21 (86%) | LA: 17 RA: 1 |
| Shapiro et al.19 | 3/4 | 56.71 (range 52–65) | 7/7 (100%) | LA: 7 |
| Erdil et al.5 | 4/7 | 55.72 (range 30–73) | 11/11 (100%) | LA: 11 |
| Rahmanian et al.20 | 10/18 | 61.3 ± 13.5 | 23/28 (82%) | LA: 24 RA: 4 |
| Study | Description of CAD and Treatment |
|---|---|
| Van Cleemput et al.16 | One patient had significant CAD of the LCx, and was considered operable. |
| Ergünes et al.17 | One patient was determined to have CAD and was treated with additional CABG. |
| Gismondi et al.18 | Three patients had significant CAD, defined as the existence of a ≥ 50% diameter narrowing of the LMCA or a ≥ 70% diameter narrowing of the other coronary arteries. |
| Shapiro et al.19 | Two patients had significant coronary obstructions. One patient had a total obstruction of the RCA and an antecedent myocardial infarction. The second had a severe lesion on the LAD. |
| Erdil et al.5 | Four patients had concomitant CAD identified. At surgery CABG was performed after the resection of left atrial myxoma in three patients. The fourth patient had a noncritical lesion in the RCA and was treated medically. |
| Rahmanian et al.20 | Six patients had significant CAD, leading to percutaneous angioplasty and stent placement in three patients, and surgical revascularization during mass excision in the remaining three patients. |
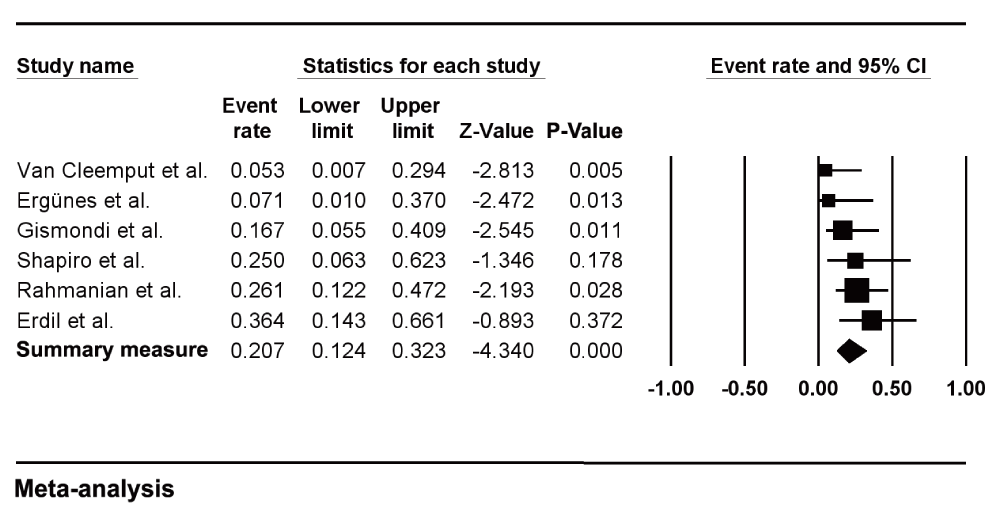
Horizontal lines represent 95% confidence intervals (CIs). Each box represents the prevalence rate point estimate, and its area is proportional to the weight of the study determined by inverse variance weighting. The diamond represents the overall summary estimate, with the 95% CI given by its width.
As shown, we found an aggregated estimate of 20.7% (95% CI 0.12 to 0.32). The prevalence rates reported across these studies varied from 5.26%16 to 36.26%5 (Figure 2), with low heterogeneity (Q-value = 5.873, P-value = 0.319, I2 = 14.86%). Egger's test (two tailed) was borderline positive for publication bias (P = 0.047). A funnel plot with Trim and Fill method is shown in Figure 3. The close observed and adjusted values of pooled prevalence suggest a small influence of publication bias.

This is a display of the study’s effect size on a logit scale against its precision for each study included in the meta-analysis. Egger's test P = 0.047. Trim and Fill method showed close observed (20.7% with 95% CI 12.4 to 32.3%) and adjusted (22.7% with 95%CI 13.0 to 36.5%) values.
There is little documented literature on the relationship between CAD and cardiac myxomas8. This article provides the first compilation of available angiographic data on adult patients with cardiac myxomas and CAD. According to this meta-analysis, the estimated prevalence of CAD in adult patients with myxomas is 20.7%, with low heterogeneity. In observational studies, Van Cleemput et al.16, Ergünes et al.17 and Gismondi et al.18 found that CAD prevalence accounted for 5.3%, 7.1% and 16.6% of adult patients with myxoma, respectively. Shapiro et al.19 found a CAD prevalence of 25% and Erdil et al.5 of 36.4% of adult patients with myxomas. However, in these studies, the number of index patients considered was small, 7 and 11, respectively. Rahmanian et al.20 evaluated 23 out of 28 adult myxoma patients with coronary angiography and found a CAD prevalence of 26.1%; nevertheless, this study enrolled the eldest patients of our series of studies (mean age, 61.3 years).
Concomitant coronary artery bypass grafting (CABG) surgery with resection of the tumor can be crucial in patients with critical coronary lesions. Erdil et al.5 identified 4 patients out of 11 with concomitant CAD, 3 of which had adjuvant CABG performed, and a fourth, which had a noncritical lesion in the right coronary artery and was treated medically. Rahmanian et al.20 also reported that in 6 out of 23 patients significant CAD was found leading to percutaneous angioplasty and stent placement in 3 patients, and surgical revascularization during mass excision in the remaining three patients. Indeed, out of the 14 patients with CAD identified in the 6 studies included in this meta-analysis, 12 (86%) were subject to, or were liable to invasive treatment.
The use of preoperative coronary angiography (CA) in adult myxoma patients is a topic of debate. Some argue that CA should only be performed in selected patients, particularly those aged > 35–40 years, with atherosclerotic risk factors, a positive anginal history or with a previous history of myocardial infarction to rule out concomitant coronary artery disease16,20–23. However, many report that there has been no significant difference in symptoms, age or prevalence of coronary risk factor distribution between myxoma patients who present with CAD and those who do not5,18,24,25. Actually, even patients without any risk factors can present with CAD25. Therefore, others suggest that all adult patients diagnosed with myxomas should undergo CA5,8,18,25,26. In fact, preoperative CA seems to be quite safe; thus far there has been no report of procedure-related complications8,16,18,24–27.
Preoperative CA can yield even more information that may prove useful intraoperatively8. Selective CA occasionally may visualize the tumor by revealing the angiographic sign of ‘tumor vascularity’, first described by Marshall et al.28, which consists of clusters of small and tortuous vessels with blood pooling and tumor blush arising from the coronary arteries supplying the tumor26,27. From the experience of Van Cleemput et al.16 and the data published by Fueredi et al.27 and Chow et al.26, angiographically visible neovascularity is prevalent in around 40% of symptomatic cardiac myxoma patients. This finding suggests a tumoral origin of the mass, however not specific. Systematic performance of preoperative CA has been recommended by some authors in an attempt to identify a large supplying vessel20.
Failure to identify and ligate these vessels may lead to a coronary-cavitary fistula29 or a “steal syndrome”, by re-directing blood from a coronary artery into a cardiac chamber, with consequent myocardial ischemia8.
Our study has limitations. As not all patients in each study had performed coronary angiography, the pooled prevalence can be overestimated by verification bias. Patients not selected to perform CA are probably those with a low risk profile. To overcome this limitation, we decided to not include those studies that reported less than 75% of patients submitted to CA. Since it is a rare condition, samples are small. Also, number of studies is small. On the other hand, a systematic review is one way to gather evidence on rare conditions. Although the publication bias test was borderline positive, the Trim and Fill method suggested small or no influence of publication bias on pooled results. Even if it is still present, prevalence could be higher, since the adjusted value is slightly higher than the observed value.
Routine preoperative angiography in all cases of these tumors is still a matter of debate. Pooled prevalence of coronary disease and the potential to disclose angiographically detectable neovascularity are arguments to advocate routine angiography. Patient management and clinical outcomes could be potentially altered, but more studies are needed to answer this question.
MCS co-conceived the study, participated in the design of the study, search strategy execution, performance of the statistical analysis, and writing the manuscript.
MSC participated in the search strategy execution, performance of the statistical analysis, and writing the manuscript.
JTN participated in the acquisition of data, performance of the statistical analysis, and writing the manuscript.
ACBS participated in the acquisition of data, performance of the statistical analysis, and writing the manuscript.
MRS co-conceived the study, participated in the design of the study, search strategy execution, performance of the statistical analysis, and writing the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Statistics
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 07 Jul 15 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)