Keywords
Gene expression, Down syndrome, iPSC, RNAseq
This article is included in the Preclinical Reproducibility and Robustness gateway.
Gene expression, Down syndrome, iPSC, RNAseq
The surprising findings by Letourneau1 and colleagues prompted us to examine our own, as yet unpublished, Ts65Dn transcriptome data for the developing and mature hippocampus in an attempt to identify GEDDs. Our data provided no evidence for the pattern reported in Letourneau et al.’s work. We first entertained the possibility that GEDDs were not present in post mitotic cells or cells undergoing neural differentiation. However, to ensure that we fully understood the published GEDD data, we examined the entire RNAseq dataset from the Letourneau manuscript, as provided publicly via the Gene Expression Omnibus.
Principal component analysis (PCA) of RNAseq replicates from the twin’s fibroblast (T1DS: twin with DS; T2N: disomic twin) revealed a great deal of variability (Figure 1A). When the datasets from Letourneau et al., are compared, in two of four cases, a closer relationship exists between the DS and disomic twin fibroblasts than for replicates from the same individual. [The datasets from Letourneau et al. are denoted by –L]. For example, one of the 2N-hFibro-L replicates clustered more tightly with a DS-hFibro-L replicate than with any of its own replicate (2N) samples.

PCA analysis of global gene expression among RNAseq replicates from the twin’s fibroblasts and iPSCs. Comparing hFibro-L replicates with themselves reveals a high degree of variability along the most significant component, PC1. In addition, there is great variability between hiPSCs-L and hiPSCs-H.
We next checked the variability of the twin’s iPSCs RNAseq data. We found an additional three RNASeq replicates (hiPSCs-H) performed by the same research group and published earlier using fibroblasts from the same monozygotic twins2. PCA analysis of these data revealed that replicates of hiPSCs-H (H for Hibaoui) clustered well together; however, they did not cluster well with the data for hiPSCs-L (Figure 1A). Altogether, PCA analysis indicated marked variability between datasets, raising the possibility that technical issues in the RNAseq samples or in their analysis compromised the Letourneau study.
To further explore the additional three hiPSCs-H RNAseq replicates, we searched for GEDDs using methods similar to those utilized by Letourneau and colleagues. Our analysis of the hiPSCs-H did not find conserved patterns indicative of GEDDs. Figure 1B shows the results for two chromosomes, as examples. The authors reported high global gene fold-change correlations between the twin’s fibroblasts and derived iPSCs. Our re-analysis found a similar high correlation value of ρ = 0.82 between the hiPSCs-L and hFibro-L. However, we found the hiPSCs-H poorly correlated with the original datasets; ρ = 0.31 between hiPSCs-L and hiPSCs-H; ρ = 0.07 between hiPSCs-H and hFibro-L (Figure 1C and Supplementary figure 1A).

(1) hiPSCs-L derived from monozygotic twins discordant for DS, Letourneau 2014 (red). (2) hiPSCs-H from the same monozygotic twins discordant for DS from Hibaoui 2014 (blue). (3) Human fibroblasts (hFibro-L) from monozygotic twins discordant for DS from Letourneau 2014 (black). hiPSCs-H lack GEDDs, while original hiPSCs-L and hFibro-L show GEDDs and high Spearman’s correlation (ρ1,3).
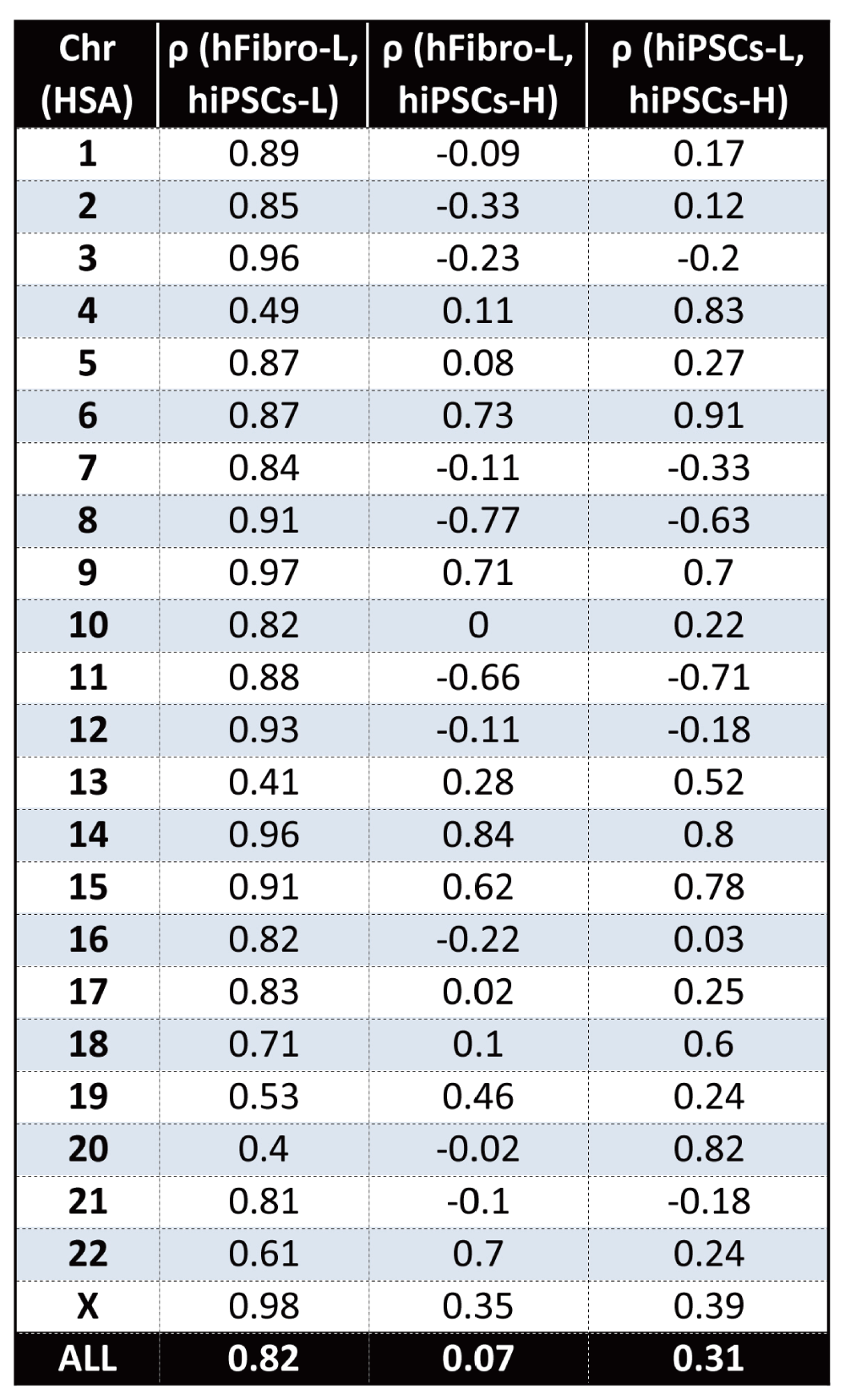
While iPSCs (hiPSCs-L) from the original study are highly similar to fibroblasts (hFibro-L) with a global correlation of ρ=0.82, hiPSCs-H show poor correlation with those samples (ρ=.07, ρ=0.31).
Conservation of GEDDs in Ts65Dn mouse model of DS were quite unexpected given that Ts65Dn mouse is segmentally trisomic (34Mb) for a portion of mouse chromosome 16 (MMU16); the segment contains about 88 mouse homologues to human genes on the long arm of HSA21; it also carries an extra copy of the approximately 10Mb centromeric segment of MMU17 that is not syntenic to any region on human chromosome 213–6. To further explore the possibility of GEDDs, RNAseq data was obtained from three replicates each from Ts65Dn and wild type mouse embryonic fibroblasts (MEFs-D) (D for Do denotes MEFs in the current study). PCA revealed tight clustering between our replicates (Figure 1D), but not those for the MEFs-L samples. While we found expected changes in gene expression in MEFs-D, analysis of MEFs-L and MEFs-D found a poor global correlation (ρ = -0.31) (Figure 1E and Supplementary figure 1B); this was also the case across all mouse chromosomes (for examples see Figure 1F and Supplementary figure 1B). In summary our findings raise serious concerns regarding the validity of GEDDs. We find no evidence for such domains in the studies on DS referenced herein or in cells from the Ts65Dn mouse model of DS.

PCA reveals little variance along the most significant component, PC1, of global gene expression among RNAseq replicates from our repeated experiments, MEFs-D. RNAseq data of mice fibroblasts from the original study, MEFs-L, do not cluster with MEFs-D.

Embryonic mice fibroblasts examined herein (MEFs-D; blue), from 2N and Ts65DN mice do not show the GEDDs reported for MEFs-L (red).
Total RNA was collected using TRIzol reagent and further purified using RNeasy mini Kit, (Qiagen) from primary mouse embryonic fibroblasts (MEFs) derived from 18.5-day-old Ts65Dn and 2N mouse using manufacturer’s instructions. RNA quality was checked using Tapestation 2200 (Agilent technologies) and quantified using Qubit instrument (Life technologies). TrueSeq stranded mRNA-seq libraries were prepared from 5 μg of total RNA (Illumina mRNA-seq kit, RS-122-2103) and sequenced using Illumina HiSeq 2500 PE-100 (sequences publically available from GEO, accession number: GSE64840). Experiments were performed in triplicate.
RNAseq data from hFibro-L, hiPSCs-L, and hiPSCs-H were downloaded from the Sequence Read Archive (SRP039348, SRP032928) and uploaded to Illumina BaseSpace for mapping (BaseSpace App v1.0, TopHat v2) and differential gene analysis (BaseSpace App v1.1, CuffLinks v2.1.1). PCA was performed using R (v3.1.0) from normalized gene count values (FPKM). Overall Spearman correlation values were calculated from locally weighted scatterplot smoothing (LOWESS) with 30% bandwidth between log2 (FC) gene expression of comparison samples, ordered by genes along each chromosome and plotted using R and custom scripts.
Custom scripts for R used to calculate Spearman correlation values are available at https://github.com/lhdo/GEDDplot
LD contributed in writing the manuscripts and all the scripts used to perform analysis of the data. NS contributed in writing the manuscript and performed all the experiments for sequencing. WM contributed in writing the manuscript. All authors have seen and agreed to the final content of the manuscript.
NIH grants NS055371 and NS24054, Larry L. Hillblom Foundation, Lumind Foundation (formerly Down Syndrome Research and Treatment Foundation), Research Down Syndrome, Thrasher Research Fund, Adler Foundation, and Alzheimer Association.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
TrueSeq stranded mRNA-seq libraries preparation and sequencing using Illumina HiSeq 2500] was conducted at the IGM Genomics Center, University of California, San Diego, La Jolla, CA.
Supplemental figure S1. A) Comparison of the gene expression fold-change profiles between T1DS and T2N in human fibroblasts and human iPS cells along all human chromosomes. (1) hiPSCs-L derived from monozygotic twins discordant for DS, Letourneau 2014 (red). (2) hiPSCs-H from the same monozygotic twins discordant for DS from Hibaoui 2014 (blue). (3) Human fibroblasts (hFibro-L) from monozygotic twins discordant for DS from Letourneau 2014 (black). hiPSCs-H lack GEDDs, while original hiPSCs-L and hFibro-L show GEDDs and high Spearman’s correlation (ρ1,3). B) Comparison of the gene expression fold-change profiles between 2N and Ts65Dn fibroblasts along all mouse chromosomes. Embryonic mice fibroblasts MEFs-D (blue), from 2N and Ts65DN mice do not show the GEDDs reported for MEFs-L (red).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 17 Jul 15 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)