Keywords
Macrophage migration inhibitory factor (MIF), malaria, birth weight, hemoglobin, Sudan
Macrophage migration inhibitory factor (MIF), malaria, birth weight, hemoglobin, Sudan
Malaria is a large public health problem in endemic tropical countries where there are over 30 million pregnancies at risk of malaria occur in Africa each year1. Malaria during pregnancy can lead to adverse outcomes (both maternal and perinatal) e.g. anemia and low birth weight (LBW)2–4. Pregnant women in different regions of Sudan are susceptible to malaria, regardless of their age and parity5. Malaria is associated with adverse pregnancy outcomes such as anemia6 and LBW7, and it is the main cause of maternal mortality8.
The sequestration of Plasmodium falciparum–infected erythrocytes and accumulation of infected erythrocytes in placental intervillous spaces is responsible for the malaria-related pathologic changes in the placenta9,10. The exact mechanism by which malaria infection and placental inflammation result in fetal growth restriction and LBW is poorly understood. However, many chemokines and inflammatory cytokines are associated with malaria infection and malaria-related LBW11.
Macrophage migration inhibitory factor (MIF) is a pro-inflammatory cytokine released from a variety of cells (T cells, monocytes, macrophages, blood dendritic cells, B cells, neutrophils, eosinophils, mast cells) and is implicated in the pathogenesis of sepsis, and inflammatory and autoimmune diseases12. MIF has been observed in the human endometrium, placental villi, cytotrophoblasts, and it has been implicated in implantation and other reproductive functions13,14. Several studies have demonstrated the role of MIF in modulating malaria severity and pathogenesis15,16. It has recently been reported that women with a positive result for placental malaria had significantly higher intervillous plasma MIF levels than women with a negative result for placental malaria17. There are few published data on MIF and placental malaria and none of them from Sudan. The current study was conducted in Medani Maternity Hospital, Central Sudan, to investigate MIF levels in women with placental malaria, and the effect – if any- on maternal hemoglobin and birth weight. This work is an addition to our previous research on malaria and its pathogenesis during pregnancy e.g. placental malaria infiltration18,19, hormones and cytokines20,21 complement, cytokines and malaria infections22,23.
A cross-sectional study was conducted during the rainy and post-rainy season (September–November) 2013 in Medani Maternity Hospital, Central Sudan which is a referral tertiary hospital. Central Sudan is characterized by unstable malaria transmission and P. falciparum is the sole malaria parasite24.
The sample size of 151 women was calculated to have 80% power and to detect a difference of 5% at α=0.05 and 10% of women might not respond or have incomplete data.
After signing an informed consent form, information on history of obstetrics, medical history, antennal attendance characteristics, and bed net use was gathered from participants using questionnaires applied by a trained medical officer. Maternal weight and height were measured and body mass index was calculated and expressed as weight (kg)/height (m)2. Newborns were weighed immediately following birth using a Salter scale and the sex of each newborn was recorded.
5ml of blood (maternal and cord) were taken and allowed to clot and centrifuged for 10 minutes at 3000 rpm and the serum was separated and stored at -20°C till the analyses.
Maternal, placental, and cord blood films were prepared and stained using 10% Giemsa. If the slides were positive; the number of asexual parasites was counted per 200 leukocytes, assuming a leukocyte count of 8000 leukocytes/μl (for thick films) or per 1000 red blood cells (for thin films). Blood films were considered negative if no parasites were detected in 100 oil immersion fields of a thick blood film, which was double-checked in a blind manner by an expert microscopist. Maternal hemoglobin levels were measured by the HemoCue hemoglobinometer (HemoCue AB, Angelhom, Sweden) and recorded.
Placental histology. The method used for placental histology was mentioned previously7,18–20. In summary, a 3cm3 placental sample was obtained from the maternal surface at a location approximately halfway between the umbilical cord and the edge of the placenta. Each biopsy sample was immediately placed in 10% neutral buffered formalin. The buffer was used to prevent formation of formalin pigment, which has similar optical characteristics and polarized light activity as malaria pigment25. Placental biopsy samples were processed and were embedded in paraffin wax and 4mm thick slides were stained with hematoxylin-eosin and Giemsa. In these slides, placental malaria infection was characterized as follows26: uninfected (no parasites or pigment), acute (parasites in intervillous spaces), chronic (parasites in maternal erythrocytes and pigment in fibrin, or cells within fibrin and/or chorionic villous syncytiotrophoblast or strom), and previous (no parasites, and pigment confined to fibrin or cells within fibrin).
Maternal and cord serum levels were measured using a human MIF ELISA kit (BIOLEGEND catalogue number 438408, Pacific Heights Blvd, San Diego, USA) by following the manufacturer’s protocol.
Data were entered into a computer using SPSS for windows (version 16.0). MIF data were not normally distributed and were compared between groups using Mann-Whitney U test. Multivariate analyses were performed using binary models for placental malaria infection as the dependent variable and linear models with hemoglobin, birth weight, and MIF (maternal and cord) levels as continuous dependent variables. Socio-demographic characteristics, education, antenatal care, residence, and placental malaria infections were the independent predictor of interest. Odds ratios (OR) and 95% confidence intervals (CI) were calculated and a P value of <0.05 was considered significant.
The study received ethical clearance from the Research Board at the Faculty of Medicine, University of Khartoum, Sudan.
The basic characteristics of the investigated women were shown in Table 1. There were no P. falciparum-positive blood films obtained from maternal peripheral blood, placenta or cord samples. Out of 151 placentae, four (2.6%), one (0.7%), 32 (21.2%) showed acute, chronic and past infection on histopathology examinations respectively, while the rest (114; 75.5%) of them showed no signs of infection.
None of the investigated factors were associated with placental malaria infection, Table 2. There was no significant difference in the median (interquartile) of maternal [5.0 (3.7–8.8) vs 6.2(3.5–12.0) ng/ml, P=0.643] and cord [8.1(3.3–16.9) vs 8.3(4.2–16.9), ng/ml, P=0.601] MIF levels between women with a positive result for placental malaria infection (n=37) and women with a negative result for placental malaria infection (n=114; Figure 1).
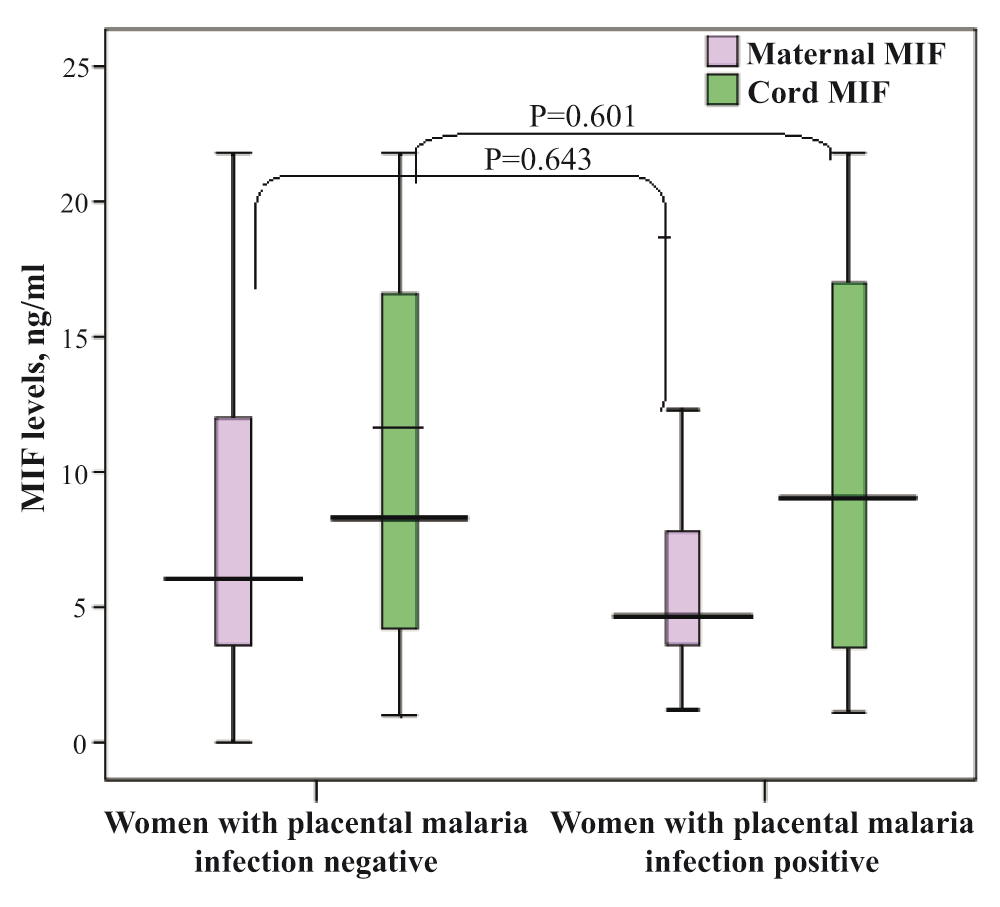
There was no significant difference in the median (interquartile) MIF levels [5.6(3.6–11.5) vs [7.3(3.0–9.7) ng/ml, P=0.516] between the maternal and cord samples.
In linear regression placental malaria was not associated with maternal MIF, hemoglobin or birth weight. Likewise MIF levels were not associated with maternal hemoglobin or newborn birth weight (Table 3 and Table 4).
There was a significant association between maternal blood/placental and cord MIF (0.473 ng/ml, P<0.001), Table 4.
The main findings of the current study were; there was no significant difference in the MIF levels in women positive for placental malaria infection and women negative placental malaria infection negative. There was no association between MIF, hemoglobin and birth weight.
This goes with previous reports where Singh et al. found no significant difference in the peripheral and cord MIF levels between women with placental malaria infections and women with placental malaria infections negative17. It is worth mentioning that in Singh’s later study the MIF levels in the intervillous space (which we did not measure) were significantly higher than the peripheral and cord levels and higher in women with placental malaria infection compared with women negative for placental malaria infection17. Furthermore, the observations of Singh et al. were based on microscopically-diagnosed placental malaria infection and in our cohort none of the women/placentae had microscopically detected malaria infections, except one that was diagnosed via histology. Yet high MIF was reported to be associated with adverse pregnancy outcome regardless of the presence of malaria infection17. Likewise, Chaisavaneeyakorn et al.27 observed high levels of MIF in the intervillous blood, compared with that in both peripheral and cord plasma and that intervillous (but not peripheral) MIF levels are associated with placental malaria among Kenyan women. Previous results obtained by Chaiyaroj and colleagues reported significantly higher MIF production by intervillous blood monocytes compared to peripheral ones and high MIF levels in placental plasma compared to paired peripheral plasma28. Increased secretion of MIF by syncytiotrophoblasts was observed previously using an in vitro system29. Generally MIF has been shown to play important roles during normal pregnancy30, as well as in preterm delivery31 and preeclampsia32 and therefore, intervillous MIF would be expected to be high.
We have previously shown that immunomodulatory hormones (cortisol), cytokines, monocytes and macrophages were implicated in the pathogenesis of malaria during pregnancy which affected pregnant women regardless of their age and parity5,7,8,18–21. Furthermore, histologic studies have shown that malaria-infected placentae have high numbers of macrophages loaded with malarial pigment and these cells could have a critical role in the clearance of the malaria parasites25. Perhaps the high levels of MIF levels observed (by the later studies) in the placenta of women positive for malaria is induced by the malaria parasites that accumulated in the placenta, with MIF helping to retain macrophages in the placenta. Interestingly, it has been shown that MIF is effective in activating macrophages to clear/remove intracellular parasites e.g. Leishmania major33.
As mentioned above, the malaria placental infections in the current study were past infections and this could explain the lack of significant difference in MIF levels. The other plausible explanation could be the submicroscopic/subpatent infections that we did not investigate in the current study. We have recently shown that in the same hospital, submicroscopic/subpatent infections that were detected by PCR rather than histology were significantly associated with low birth weight7.
The current study failed to show a significant association between maternal blood/placental and cord MIF levels, placental malaria, maternal hemoglobin or birth weight.
F1000Research: Dataset 1. Raw dataset for Eltayeb et al., 2015 ‘Macrophage migration inhibitory factor and placental malaria infection in an area characterized by unstable malaria transmission in Central Sudan’, 10.5256/f1000research.7061.d10203934
RE and IA coordinated and carried out the study. NEB and AA participated in the statistical analysis. EME and AAM participated in the clinical work and conducted the laboratory work. All the authors have read and approved the final version of this manuscript.
Authors would like to thank the women who were involved in the study and the midwives and the nursing staff of the Medani Hospital.
Data collection questionnaire.
Questionnaire was applied by a medical professional to participants in the study. ANC: antenatal care; HB: haemoglobin; WT: weight
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 16 Sep 15 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)