Keywords
Nitrofurantoin, urinary tract infection, pulmonary injury nitrofurantoin-induced interstitial lung disease, NIILD
Nitrofurantoin, urinary tract infection, pulmonary injury nitrofurantoin-induced interstitial lung disease, NIILD
A previously healthy 83-year-old Caucasian woman presented with progressive dyspnoea. She was a 50 pack per year ex-smoker. She suffered from recurrent symptomatic urinary tract infections (UTIs) and was prescribed long-term nitrofurantoin 50 mg daily for prophylaxis by her GP.
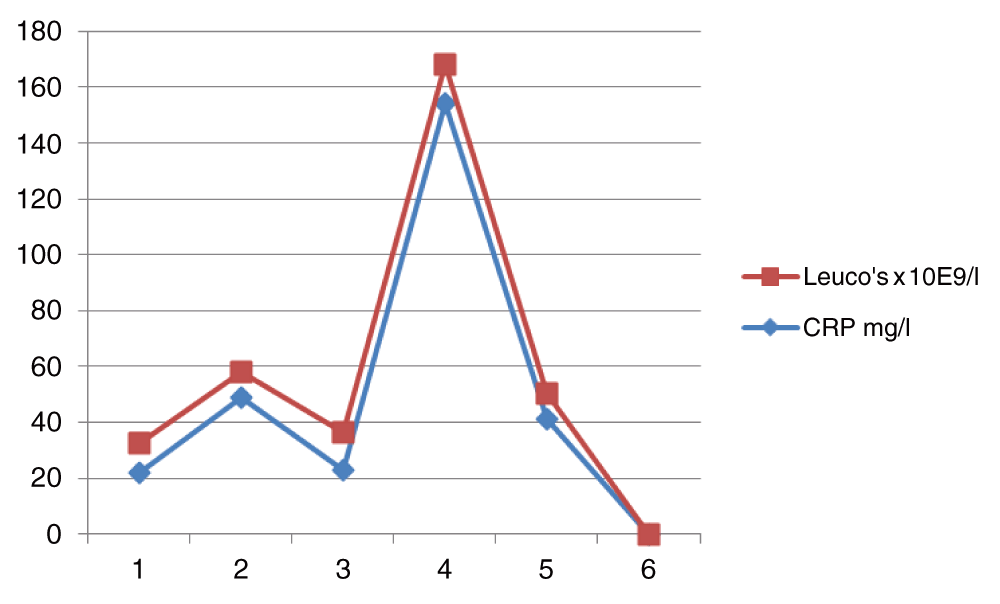
On examination she appeared dyspnoeic. She was afebrile and normotensive with respiratory rate of 31 per minute and oxygen saturation was 91% while receiving supplementary oxygen at a flow of 5 liter per minute. Respiratory examination revealed fine inspiratory crackles throughout both lungs. Arterial blood gas showed hypoxia with PaO2 5.4 kPa (without oxygen suppletion), PaO2 8.3 kPa (with 5 liter/min oxygen suppletion) and complete compensation of respiratory acidosis with pH 7.37, and PaCO2 6.9 kPa on 5 liter oxygen suppletion with base excess 2.9 mmol/l [Figure 1]. Laboratory findings showed increased leucocytes and C-reactive protein level [Figure 2]. Auto-immune laboratory findings including the antinuclear antibody and rheumatoid factor tests were negative. Chest X-ray revealed diffuse bilateral interstitial infiltrates [Figure 3]. CT scanning of the chest showed widespread ground-glass appearance with organizing pneumonia [Figure 4]. Initial treatment with co-amoxiclavulanic acid was started at a dose of 1.2 gram 4 times daily.
Nitrofurantoin was subsequently stopped and prednisolone treatment at 30 mg OD was initiated. She had a short hospital course of 12 days and was finally discharged without long term oxygen treatment. Follow up chest X-ray before discharge and after withdrawal of nitrofurantoin (Figure 5) showed marked improvement compared to the X-ray upon admission.
The patient was seen after two months during outpatient control; her symptoms had improved dramatically and a follow-up chest X-ray showed further normalization.
The differential diagnosis of nitrofurantoin induced interstitial lung disease (NIILD) includes pulmonary edema, cryptogenic organizing pneumonia and idiopathic interstitial pneumonias1. The auto-immune markers tested, antinuclear antibody and rheumatoid factor, were negative indicating a reaction to nitrofurantoin rather than an underlying systemic pathology.
Prompt resolution following the discontinuation of nitrofurantoin further supports the diagnosis2,3. The diagnosis is based on the history of nitrofurantoin use and the absence of another explanation for the patient’s symptoms and radiographic abnormalities.
Discontinuation of nitrofurantoin results in the regression of symptoms and radiographic abnormalities3. Systemic corticosteroids are occasionally administered and it remains unclear how much corticosteroids contribute to improvement beyond drug cessation alone4.
Long-term use of nitrofurantoin as prophylaxis for UTIs can cause serious pulmonary side effects. Our patient received antibiotics and corticosteroids because of diagnostic uncertainty. This may represent frequent clinical practice, however there are no specific symptoms to separate NIILD from other interstitial lung diseases1.
Written informed consent for publication of clinical details and clinical images was obtained from the next of kin.
Suhail Basunaid: data collection, drafting and revising the manuscript for intellectual content. Pilate Helena: clinician assessing and looking after the patient. Schoutteten Melanie: clinician assessing and looking after the patient. Sprooten Rooy: the chief clinician looking after the ward.
At the completion of this case study, I am very thankful for Dr. Rohde Gernot’s help, and without his support this case study would have never come into its present form.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 01 Apr 15 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)