Keywords
EFK-1, EEF-2, translation regulation, Caenorhabditis elegans
This article is included in the Antibody Validations gateway.
EFK-1, EEF-2, translation regulation, Caenorhabditis elegans
Protein synthesis determines the cellular proteome and its regulation is pivotal to maintaining cellular homeostasis. This process is divided into three mains stages: initiation, elongation and termination1. The most studied step in eukaryotes is translation initiation and its regulation is critical to cell survival under stress conditions. Elongation regulation is also important in modulating translation. During this process, eukaryotic elongation factor 2 (eEF2) catalyzes the translocation of peptidyl-tRNA from the A site to the P site on the ribosome. The phosphorylation of eEF2 at threonine 56 by eEF2 kinase (eEF2K) inhibits its binding to ribosome and thus its activity2–5 in stress- or starvation-related conditions6–8. eEF2 kinase is an atypical α-kinase, normally dependent on Ca2+ ions and calmodulin, and apparently has only one substrate, the elongation factor eEF29. eEF2 phosphorylation by eEF2K results in a reduction in translation rate. C. elegans processes eEF2 and eEF2K orthologues, named as EEF-2 (94.8 kDa) and EFK-1 (87.8 kDa), respectively. As in other eukaryotes, the Thr56 residue and adjacent sequences in EEF-2 are conserved. The only described data about efk-1 shows that it is important to nutrient deprivation resistance10. My lab studies the role of efk-1 in various aspects of C. elegans survival and the simplest way to measure its activity is to determine the EEF-2 phosphorylation status. Here we show that phospho-eEF2 antibody from Cell Signaling Technology Inc., specific to vertebrates, has excellent reactivity against C. elegans phospho-EEF-2 and this property is preserved after freeze/thaw cycles.
The standard C. elegans strain N2 Bristol (Caenorhabditis Genetic Center – CGC- wild isolate) and knockout strain efk-1 (CGC#RB2588), were maintained at 15°C and propagated on E. coli strain OP50 (CGC) using established procedures11,12. Gene knockout was verified by Polymerase Chain Reaction (PCR) using GoTaq® DNA polymerase (Promega) and specific primers to efk-1 (Fw- ATGACGATCGACACAACAAA/Rv- AGATCACCAACTCCTTGAATATCG) and act-1 (Fw-ACCATGTACCCAGGAATTGC/Rv- TGGAAGGTGGAGAGGGAAG) (Figure 1).
Worms (n= ~10) were collected in M9 buffer12 and washed three times by centrifugation at 1000 rpm for 1 min (RT) in M9 Buffer to remove bacterial cells. Worm pellets were heated (95°C) in 2X sodium dodecyl-sulphate (SDS) sample buffer (62.5mm Tris-HCl pH 6.8, 25% glycerol, 2% SDS, 0.01% bromophenol blue, 100mM DTT) for 10 min. Samples were loaded in a gradient gel (8–16% - GE Healthcare Lifesciences) using the ECL® gel box system (GE Healthcare Lifesciences) at 150V (as per manufacturer’s protocol). Separated proteins were transferred to an ECL®-Hybond (GE Healthcare Lifesciences) membrane using semi-dry transfer system (Bio-Rad). The membrane was blocked with 5% bovine serum albumin (BSA) in Tris-Buffered Saline (TBS-50mM Tris, 150mM NaCl) containing 0.5% Tween 20 (TBS-T) for 1 h at room temperature, then incubated overnight at 4°C with primary monoclonal antibodies raised against vertebrate phospho-eEF2 (Thr56) (1:1000 in TBS plus 2,5% BSA – 94.8 kDa - Cell signaling Technology Inc. #2331 (Danves, MA – USA) – reactivity: human, mouse, rat, hamster, monkey, chicken) and a monoclonal anti-α-Tubulin produced in mouse (1:1000 – Sigma-Aldrich Co. LCC #T6047 - reactivity: human, chlamydomonas, African green monkey, chicken, mouse, bovine, rat, kangaroo rat, sea urchin) simultaneously. After washes, membrane was the incubated with secondary anti-rabbit/anti-mouse IgG horseradish peroxidase (HRP) antibody for 40 minutes at room temperature, subject to new washes and incubated with anti-mouse IgG HRP antibody (HRP – 1:2000 – Sigma-Aldrich Co. LCC). Signal detection was performed with Luminata® forte HRP Western substrate (Millipore). Mixed primary antibodies were stored at -20°C until needed and thawed at room temperature when necessary. Reagents are listed in Table 1 and Table 2 and the WB protocol is given in Table 3.
Using the described protocol, I could specifically detect the phosphorylated form of EEF-2, eEF-2 orthologue in C elegans since in efk-1 knockout worms, used as a negative control of efk-1 activity, there is no equivalent signal in western blots (Figure 2 and Figure 3). Detection of α-tubulin was used as a western blot load control. Some differences can be observed in α-tubulin detection when comparing lanes (Figure 2 and Figure 3), despite the use of approximately the same number of nematodes. This can be explained by the fact that some worms remain attached to the pipette tip after washes, and this effect can be circumvented adding 0.01% Triton X-100 (Sigma Aldrich) to C. elegans wash buffer (M9 buffer). In addition, both antibodies (directed to target and load control) can be used and detected simultaneously, reducing analysis time (Figure 3).
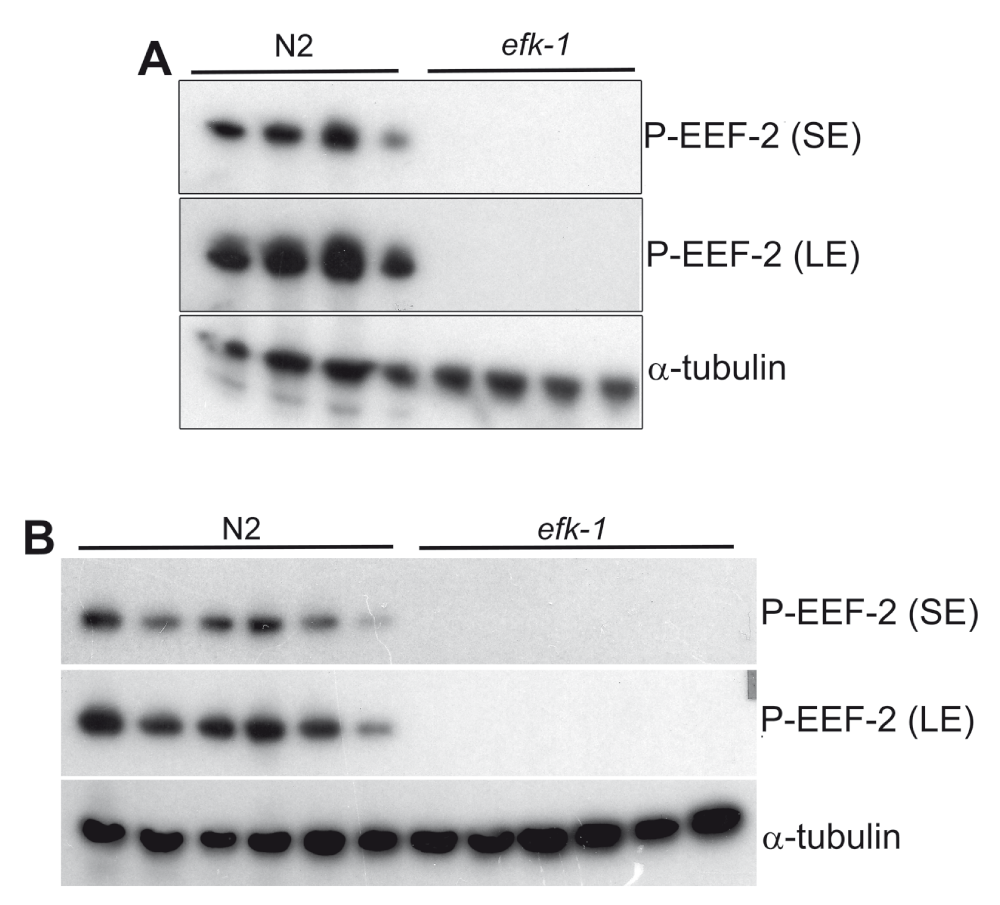
Western blot showing that phospho-eEF2 antibody recognizes C. elegans (N2 Bristol) phospho-EEF-2, since protein detection signal is absent in efk-1 knockout worms. A) phospho-EEF-2 detection at the first time antibody dilution in TBS-T-BSA. B) phospho-EEF-2 detection after five freeze/thaw cycles of the antibody in TBS-T-BSA. SE- short exposure (5 sec); LE-long exposure (1 min).
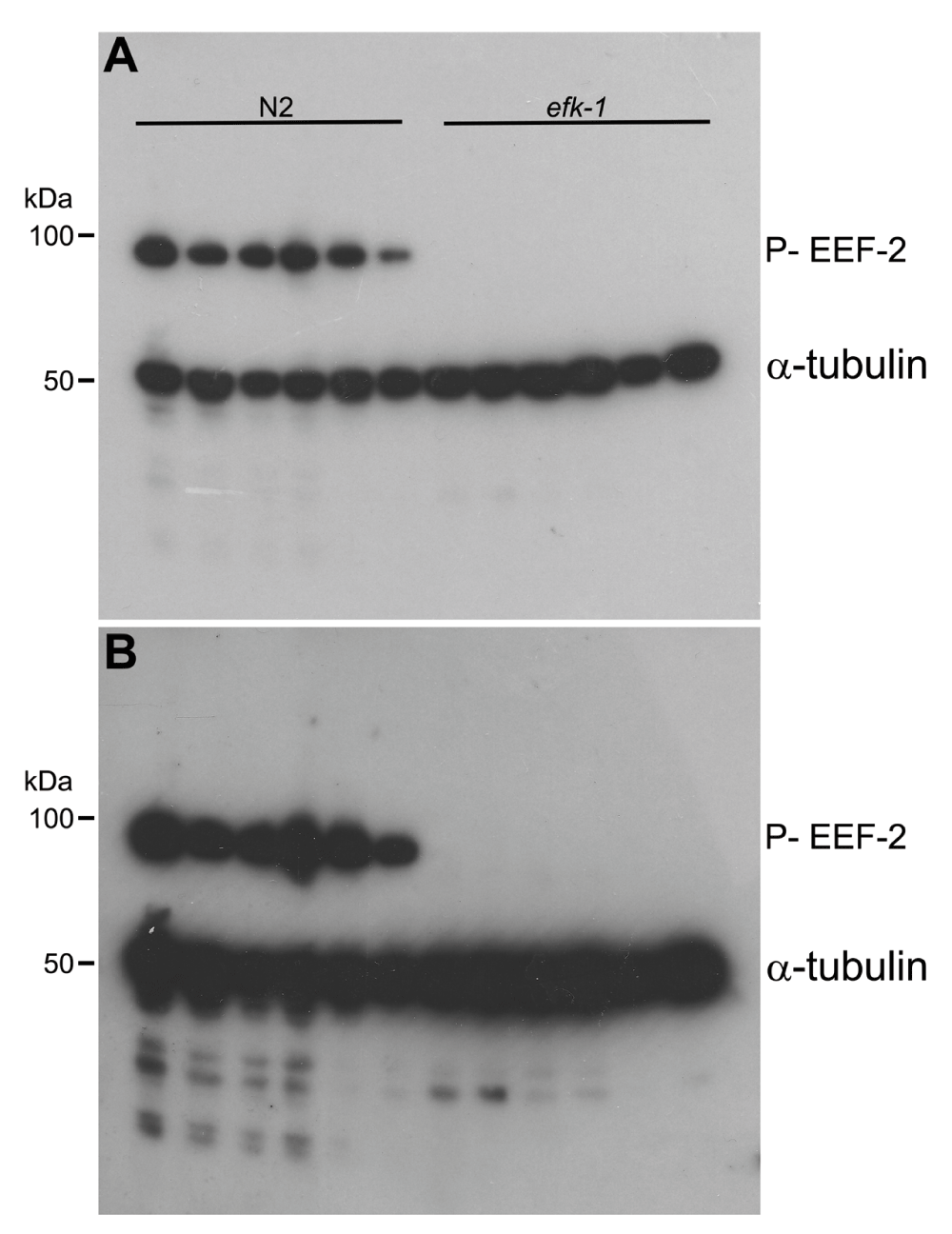
Western blot showing that phospho-eEF2 and α-tubulin antibodies can be incubated and developed simultaneously. efk-1 knockout worms were used as negative control to phospho-EEF-2 detection showed in wild-type worms (N2). A) Western blot short exposure (1 min). B) Western blot short exposure (3 min). Indicated molecular weight based on Precision Plus Protein™ Dual Color Standard (Bio-Rad).
EEF-2 phosphorylation is not observed in efk-1 knockout C. elegans, indicating that EFK-1 is the sole EEF-2 kinase in my tested conditions. Surprisingly, primary antibodies diluted in TBS-T-BSA (either phospho-eEF2 and α-tubulin, together or individual dilutions), stored at -20°C, can be reutilized at least five times (by thawing at room temperature) without losing specificity/reactivity (Figure 2B).
In this work, I show that vertebrate-directed phospho-eEF2 antibody specifically recognizes C. elegans orthologue phospho-EEF-2. This finding is very useful to those who work with translation elongation regulation using C. elegans as a model and I recommend this antibody to detect EFK-1 activity in C. elegans.
This work was supported by CNPq – Centro Nacional de Desenvolvimento Cientifico e Tecnologico, Capes – Coordenaçao de Aperfeiçoamento de Pessoal de nível Superior, and PRPq-UFMG – Pro-reitoria de Pesquisa da Universidade Federal de Minas Gerais.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The author thanks the Caenorhabditis genetic stock center (CGC) funded by NIH Office of Research Infrastructure Programs (P40 OD010440) for the C. elegans strains and Dr. Beatriz A. Castilho for the phospho-eEF2 antibody.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 25 Sep 15 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)