Keywords
Glyco-biology, hair follicle biology, alopecia, hair cycling, glycan,
Glyco-biology, hair follicle biology, alopecia, hair cycling, glycan,
The hair follicle is a true paradigm of mesenchymal-epithelial interaction. From early morphogenesis to a fully formed organ, the hair follicle life-cycle is controlled by a dialog between mesenchymal and epithelial compartments1. However, this dialog relies on a delicate balance between conflicting and/or opposing influences.
With respect to hair follicle morphogenesis, the reaction-diffusion model explains how slowly diffusing inducers and rapidly diffusing inhibitors orchestrate, through local activation and at distance inhibition, the hair follicle patterned formation. Indeed, the seminal work of A. Turing2 has been recently confirmed through a formal identification of morphogen activator-inhibitor couples, such as Wnt/DKK13 (Figure 1) and EDAR/BMP4.

(A) Wnt morphogen stimulates its own synthesis as well as that of Dkk1, its inhibitor. Wnt diffuses slowly while Dkk1 diffuses rapidly. (B) As a result, in a periodic way, Wnt concentration is higher than that of DKK1, and a hair placode can develop. (C) The reaction-diffusion process thus explains the patterned distribution of hair follicles at the surface of the scalp.
Considering its dual mesenchymal and epithelial origin, the hair follicle can be considered a composite organ, with a concentric structure. Dermal and epithelial compartments interact with each other and are characterized by specific differentiation programs. Opposing signaling pathways concur to control the unique behavior of human hair follicle and maintain its unique intrinsic homeostasis. As the activity of diffusible factors, such as growth factors and morphogens, can be modulated by glycans, their possible role in hair growth control must be taken into account.
The hair follicle is the only organ in mammals that sequentially and repeatedly transits from a phase of active fiber production (anagen) to a resting phase (telogen), through rapid phases of tissue regression (catagen) and regeneration (neogen). A recently published comprehensive guide describes most of the morphological and immunohistological markers that characterize the different stages of the human hair follicle cycle and the intense tissue remodeling events which take place5. Of note, hair follicle regeneration relies on the cyclical activation of stem cells6. In the human hair follicle, these stem cells are harbored within two distinct reservoirs7,8, one of them bathing in a hypoxic environment9. Instead of a cyclical behavior with an intrinsic automaton, the human hair follicle exhibits a stochastic behavior, the probability of duration of each phase fitting with a lognormal equation10. A new concept (Figure 2) postulates the existence of a bi-stable equilibrium11 which controls human hair follicle dynamics, including an active steady state (the anagen stage) and a resting steady state (the telogen stage), the transition between these two steady states involving either a degradation phase (the catagen phase) or a neo-morphogenesis phase (the neogen phase). It is now believed that mesenchymal and epithelial oscillators control the stochastic autonomous switching between these two steady states12,13. The transition phases are both controlled by a complex and dynamic network of interacting activators and inhibitors, diffusible morphogens, and growth factors of opposite influences14. Of note, however, extrapolating from results only obtained in rodents must be approached with caution, since major differences exist between human and mouse hair follicles in terms of phase duration, synchronicity, tissue remodeling, stem cell reservoirs, and so on.
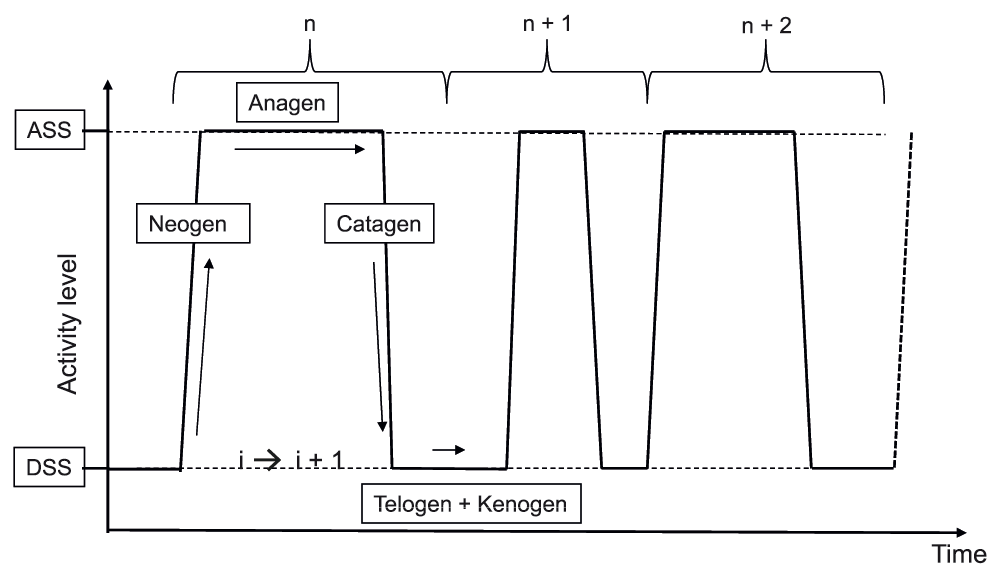
An active steady state (ASS) of fiber production (anagen) and a dormant steady state (DSS) (telogen/kenogen) are interspaced by short-lasting phases of regression (catagen) and neomorphogenesis (neogen).
During the active steady state, hair fiber production results from a finely, timely, and spatially tuned choreography of gene expression, which is highly sensitive to stimulatory and inhibitory signals. A number of signaling pathways15, cytokines16,17, neuropeptides18, hormones19–22, prostaglandins23, and growth factors24 are known to modulate the duration of the active steady state of the hair follicle (Figure 3). For example, while insulin-like growth factor (IGF)-1 is required for anagen maintenance25,26, fibroblast growth factor (FGF)-5 appears to be a crucial regulator of hair length in humans27, as a strong inducer of the catagen phase. Moreover, the human hair follicle is endowed with an autonomous androgen metabolism28, a strict dependence on arginine29, polyamines30, and glucose31 for growth, and a specific immunological response32. The hair follicle is also endowed with a full prostaglandin metabolism and a complex network of prostaglandin (PG) receptors33,34. Recent data suggest that a delicate equilibrium between PGE2/PGF2a on the one hand and PGD2 on the other hand controls the duration of the active steady state. PGE2/PGF2a promotes hair growth maintenance, while PGD2 inhibits it and triggers anagen to catagen transition35. Finally, re-evaluating the mechanisms by which agents such as cyclosporine A36 or JAK-STAT inhibitors37 promote human hair growth might help to identify new key genes and pathways involved in the control of hair growth.
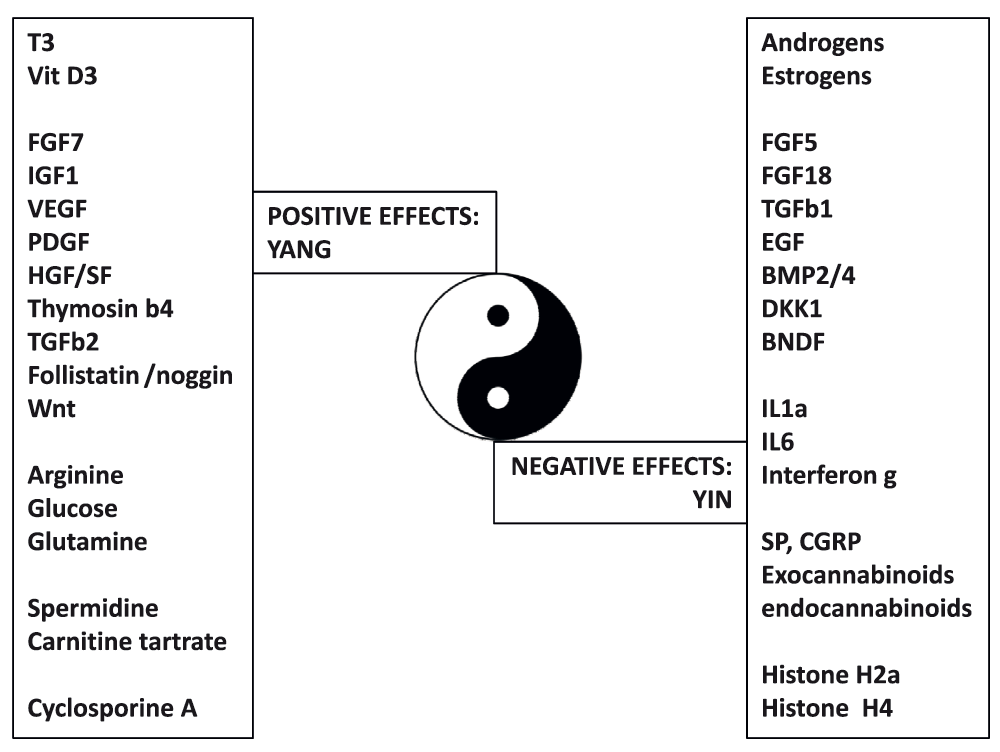
Summary of diffusible factors having positive (Yang) or negative (Yin) effects on hair growth and cycling.
Besides the active steady state, new data demonstrate that the resting steady state is not as quiescent as suspected and can be divided into a refractory period and a permissive period. Indeed, during the telogen phase, the follicle is under the influence of factors that would repress the onset of the neogen phase and factors that would trigger it. Specifically, a strong expression of bone morphogenetic protein (BMP) and FGF-18 defines the refractory period, during which the neogen onset is prevented. The progressive increase in the production of BMP antagonist noggin, Wnt/Fzz/b-catenin pathway activators, and transforming growth factor (TGF)-β2 then reaches a critical threshold that shifts the telogen follicle to a competency status, receptive to FGF-7, secreted by the nearby dermal papilla, and, ultimately, triggers the onset of the neogen phase38.
It is clear from the above that the complex and rhythmic behavior of the human hair follicle is under the control of multiple, intricate pathways with opposing influences. In this respect, the interdependence and complementary roles of these influences allow us to propose that the hair follicle is a true paradigm of a “Yin Yang” type duality and harmony. However, in our opinion, the fine tuning of these influences cannot solely rely on the timely and spatially controlled gene expression, but also on glycans, “the third revolution in evolution”39. Glycans are endowed with such a huge molecular diversity that they can be considered the third language of life, after DNA and proteins.
Linear or branched oligosaccharides can be attached to a protein backbone via O-(serine/threonine) or N-(asparagine) linkages. They form the large class of N-Complex type glycans. Glycosaminoglycans are linear copolymers of 6-O-sulfated disaccharide units which define them as chondroitin, dermatan, keratin, or heparin sulfates. Proteoglycans have one or more glycosaminoglycan side chains attached to a core protein. Glycosaminoglycans, proteoglycans, and glycan moieties of glycoproteins have long been known to play important roles in the maintenance of protein conformation and solubility, protection against proteolytic degradation, mediation of biological activity, intracellular sorting and externalization, and embryonic development and differentiation40–45. The distribution of proteoglycans in the human hair follicle was originally described in the early 1990s, namely for chondroitin sulfate, dermatan sulfate, and heparin sulfate proteoglycans46, for syndecan 1, perlecan and decorin47, and for versican48. Thanks to the availability of new immunological tools, the distribution of proteoglycans in the human hair follicle has been further refined49 (Figure 4), highlighting a complex, dynamic, and regionalized network of proteoglycans. With respect to cell surface complex type N-glycans, the use of specific fluorescently labeled lectins (saccharide-binding proteins) revealed a differential N-glycan composition among the different hair follicle compartments50–52 (Figure 5).

Diagram shows the distribution of versican, perlecan, syndecan 1, aggrecan, biglycan, and heparan sulfate proteoglycans in the different hair follicle compartments. BM, basement membrane; CTS, connective tissue sheath; IRS, inner root sheath; ORS, outer root sheath.

Distribution of N-glycans identified by their reactivity with fluorescently labelled Pisum sativum agglutinin (PSA), wheat germ agglutinin (WGA) and Ulex europeus agglutinin (UEA) in both skin and hair follicles. PSA mainly decorates the dermal compartments of skin and hair follicles, while WGA decorates both dermal and epithelial compartments. UEA only decorates the epidermis stratum granulosum and the hair follicle IRS.
What could be the role of these glycans? It has been known for quite a long time that growth factor activation could be regulated by proteoglycans53,54 and that heparan sulfate proteoglycans were involved in fine-tuning mammalian physiology55 and in cell signaling during development56. With respect to key regulators of hair follicle growth and cycling, syndecans modulate Wnt signaling cascades57, the glycosaminoglycan chains of proteoglycans shape Hedgehog gradients and signal transduction58, and O-linked glycosylation controls Notch1 interaction with its cognate Delta-like 4 receptor59. Decorin, a small leucine-rich proteoglycan, directly modulates TGF-β, epidermal growth factor (EGF), IGF-1 and hepatocyte growth factor (HGF) signaling, all known actors of hair follicle cycling60, and appears to act as an anagen inducer61. Altogether, these recent results designate glycans as long time ignored key players in hair growth control. But, on top of that, enzymes can further modulate the biological activity of these glycans. For example, fucosyl transferase is absolutely required for Notch activity, and disruption of fucosyl transferase expression in murine hair follicle lineages results in aberrant telogen morphology, a decrease of bulge stem cell markers, a delay in anagen re-entry, and dysregulation of proliferation and apoptosis during the hair cycle transition62. With respect to proteoglycans, heparanase (an endoglycosidase that cleaves heparin sulfate) was found expressed in the outer root sheath of murine hair follicles and identified as an important regulator of hair growth through its ability to release heparin-bound growth factors63. In the human hair follicle, however, heparanase was found located in the inner root sheath. Its inhibition provoked an immediate transition from anagen to catagen64. In this case, the HPSG/heparanase network appears to be a key controller of internal hair follicle homeostasis.
Finally, extracellular sulfatases appear to be critical regulators of heparin sulfate activities. Sulf1 and Sulf2, by removing glucosamine-6S groups from specific regions of heparan sulfate chain, modulate (a) Wnt interaction with its cognate receptor Frizzled, (b) BMP signaling by releasing BMP antagonist Noggin, and (c) FGF-2 ability to form the functional FGF-2-HS-FGFR ternary complex65,66. Of note, TGF-β1, by inducing Sulf1 expression67, might indirectly modulate Wnt, BMP, and FGF-2 activities, which could explain its inhibitory effect on hair growth. From a clinical point of view, alterations of glycosaminoglycan degradation provoke mucopolysaccharidoses and abnormalities in hair morphology68, which can be reversed by appropriate enzyme replacement therapy69.
The hair follicle is clearly endowed with a unique behavior. Its bi-stability and the intense remodeling processes that it provokes rely on the permanent dialog between opposing and complementary influences, impacting all follicle compartments. From this interdependent duality, one can easily understand that an optimal way to describe the complex equilibrium which controls hair follicle homeostasis is the concept of “Yin Yang”. Until recently, the understanding of hair growth mainly relied on deciphering the patterns of gene expression within the different hair follicle compartments throughout the hair cycle70,71. From now on, the fine-tuning of the activities of growth factors and morphogens by the modulating effects of glycans will also have to be taken into consideration.
From a prospective point of view, it is likely that a better understanding of hair diseases, and more specifically the role of inflammation and immune response in the development of alopecia areata72 and androgenetic alopecia73, will likely provide further insights into the role of the so-called immune privilege74 in hair growth control. Moreover, with the advent of mature metabolomics technologies75 coupled with in vitro human hair growth technology76, one can predict that this integrative approach will permit us to identify these key metabolic pathways sustaining normal hair growth.
I thank Ms E. Debecker (L’Oréal R&I) for her expert assistance in lectin labeling experiments.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 08 Feb 16 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)