Introduction
Allogeneic haematopoietic stem cell transplantation (HSCT) is used to treat malignant and non-malignant haematological conditions1. In primary immunodeficiency, the aim following HSCT is to achieve complete and long-lasting immunoreconstitution (IR) with a diverse T cell receptor (TCR) repertoire, providing adequate adaptive T lymphocyte immunity2. Delayed or persisting immunodeficiency is associated with significant morbidity and mortality with increased risk of infection, relapse, and development of secondary malignancies3,4. Potential strategies to boost thymic function and promote faster and complete IR, particularly in older patients who exhibit reduced thymic function inherently due to aging, have garnered much interest to improve patient outcome. Such approaches include the use of Fgf7 or sex steroid hormone inhibition, which have been shown to protect thymic epithelial cells (TECs) and improve thymopoiesis in experimental models5.
Effect of graft-versus-host disease on T lymphocyte immunoreconstitution
Conditioning given prior to HSCT results in an inevitable period of aplasia with obliteration of innate and adaptive immune responses, subjecting the patient to a period of increased risk of infection and other complications until the stem cells engraft and reconstitution of the immune system compartments ensues. Rebuilding of innate immunity, including monocytes, granulocytes, and epithelial barriers, occurs relatively quickly following HSCT, providing protection against bacterial and fungal infections6. In contrast, T lymphocyte reconstitution is lengthier and more complex, involving two pathways6–8. Peripheral thymic-independent expansion of surviving host T lymphocytes and/or transferred donor T lymphocytes provides a degree of immediate T lymphocyte immunity but of limited diversity and permanency5. Complete IR following lymphodepletion requires durable de novo thymic regeneration of naïve T lymphocytes from donor progenitor cells with a broad TCR repertoire, which requires a functioning and structurally intact thymus8,9. These naïve T lymphocytes (termed recent thymic emigrants [RTEs]) can be measured quantitatively by identification of surface markers such as CD45RA and CD31 using flow cytometry and by determination of TCR excision circle (TREC) levels. TRECs are circular pieces of DNA produced as a consequence of TCR α and β chain formation, and quantification of TREC content in T lymphocytes provides a practical and accepted measurement of thymic output10. The quality of the T lymphocyte compartment can be assessed by measuring TCR diversity, as this is almost completely reflective of the naïve T lymphocyte population11. This can be done using flow cytometry, spectratyping of the complementarity determining region 3 (CDR3), and nucleotide sequencing. Flow cytometry is widely available and cheaper and results can be obtained quickly12. Spectratyping analyses the lengths of the hypervariable region CDR3 in each Vβ family using real-time polymerase chain reaction13,14. Compared to flow cytometry, spectratyping provides more detailed resolution of TCR diversity; however, there is no accepted single standardised method of analysing data at present, and this technique gives equal weighting to all Vβ families measured, independent of how many genes they contain13. Nucleotide sequencing of DNA CDR3 regions provides even more in-depth analysis but is expensive and, although evolving, is not widely available at present15. Thymic damage disrupts normal T lymphocyte ontogeny, resulting in reduced export of RTEs and a distorted TCR repertoire, negatively impacting on IR and clinical outcome5,16–18.
Graft-versus-host disease (GvHD) is a leading cause of post-HSCT mortality19,20. Acute (a)GvHD is mediated by alloreactive mature donor T lymphocytes, which attack disparate recipient antigens, resulting in a harmful inflammatory response and tissue injury21. Elucidation of aGvHD pathophysiology is based on experimental models20: (1) damage to host tissue by conditioning regimens, underlying disease, and/or infections increases pro-inflammatory cytokines activating host antigen-presenting cells (APCs); (2) donor T lymphocytes recognise the disparate alloantigens on activated host APCs and become activated, proliferate, differentiate, produce further inflammatory cytokines, and migrate to target organs; (3) effector cells, primarily cytotoxic T lymphocytes and natural killer (NK) cells, and soluble effectors cause apoptosis of target cells.
Although aGvHD principally involves the skin, gastrointestinal tract, and liver, the thymus is also a primary target, resulting in disruption of thymic architecture with loss of cortico-medullary demarcation, alteration of TEC subpopulations, and depletion of thymocytes22–24. The precise mechanisms behind aGvHD-induced thymic injury in humans remain incompletely understood, but experimental models have helped delineate the underlying cellular and molecular mechanisms22. TECs are initiators and targets of thymic aGvHD, capable of activating alloreactive donor T lymphocytes independently of APCs, leading to secretion of interferon gamma (IFNγ) and triggering signal transducer and activator of transcription 1 (STAT1)-induced apoptosis of cortical and medullary TECs9. The resulting disruption of architecture and organisation of the thymic microenvironment with thymic atrophy disturbs the normal signalling required for immature thymocyte development, particularly at the triple-negative proliferative stage and with increased apoptosis of double-positive cells22,25,26, resulting in impaired lymphopoiesis and reduced thymic export (Figure 1)11,27. Acute GvHD also impairs the thymic-independent pathway with reduced expansion of transferred mature donor T lymphocytes, possibly due to loss of peripheral T lymphocyte niches28.
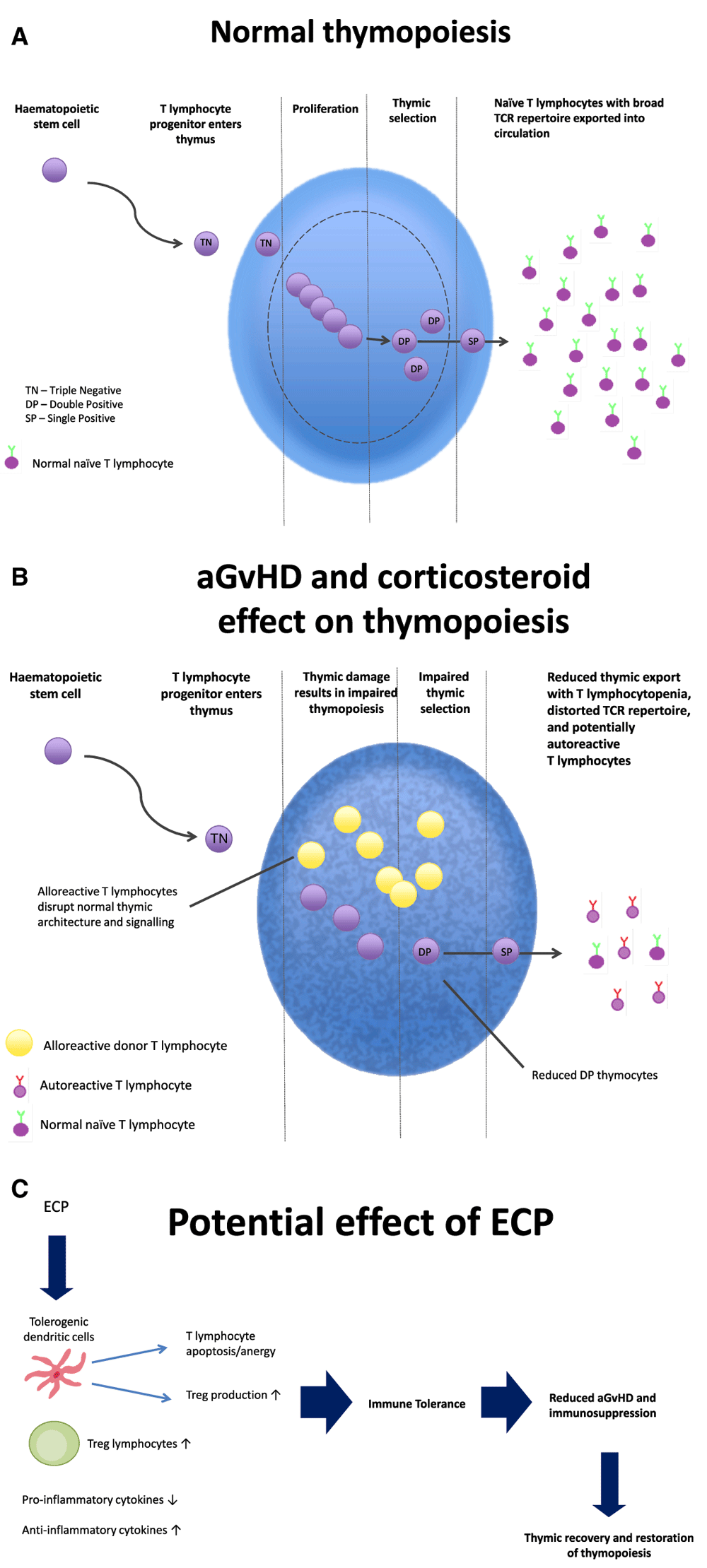
Figure 1. Normal thymopoiesis, effect of acute graft-versus-host disease (aGvHD) and corticosteroids on thymic function, and the potential effect of extracorporeal photopheresis (ECP) allowing thymic recovery.
Thymic damage occurs secondary to allogeneic T lymphocytotoxicity during aGvHD, corticosteroid-mediated damage, and other non-selective T lymphocyte-suppressive agents used in the treatment of aGvHD, causing impaired thymopoiesis (A), with reduced thymic export and a distorted T cell receptor (TCR) repertoire with potentially autoreactive thymocytes escaping negative selection (B). ECP, by promoting immune tolerance and enabling reduction and cessation of conventional immunosuppression, may allow thymic recovery, resumption of normal thymopoiesis, and complete and long-lasting immunoreconstitution post-haematopoietic stem cell transplantation (C). Abbreviations: Treg, regulatory T lymphocyte; DP, double positive.
A distorted TCR repertoire is observed in patients with aGvHD10. Disparate donor and recipient major histocompatibility complex (MHC) complexes disturb thymic positive and negative selection, impacting on TCR selection, resulting in thymocytes escaping negative selection, and increasing the survival of autoreactive T lymphocytes29–32. Thus, aGvHD is detrimental to the quantity and quality of T lymphocyte recovery. The thymus is particularly sensitive to aGvHD, with thymic output being significantly affected, even in grade 1 disease11. Subclinical thymic aGvHD may have an underappreciated adverse effect on the reconstitution of adaptive immunity, causing ongoing infections and incomplete IR post-HSCT.
Corticosteroid treatment of acute graft-versus-host disease
Corticosteroids, with potent immunosuppressive and anti-inflammatory effects, are the first-line treatment for aGvHD, but a complete response is witnessed in only 25–50% of patients33. Short, intensive courses of corticosteroids induce thymic involution, causing a profound reduction in naïve T lymphocyte production, although with complete recovery following cessation34. However, the precise effects in human thymus and of long-term corticosteroid use are unknown. There is no consensus for second-line therapy for steroid-dependent/-refractory aGvHD, which usually involves the intensification of systemic immunosuppression with a plethora of therapeutic agents that non-selectively target T lymphocytes35,36. Second-line options include mycophenolate mofetil, anti-tumour necrosis factor alpha antibodies, or mammalian target of rapamycin (mTOR) inhibitors. The use of mesenchymal stromal cells has also been advocated, with mixed success, in part because the product is a cellular therapy and it is difficult to ensure consistency of the cellular content37–39. Acute GvHD and immunosuppressive treatment concurrently impair thymopoiesis, subjecting patients to further risk of infection, relapse, and development of secondary malignancies, as well as associated toxicity40,41. A targeted therapy for aGvHD without systemic immunosuppression and that allows thymic recovery is needed42.
Extracorporeal photopheresis
Extracorporeal photopheresis (ECP) exposes apheresed mononuclear cells to 8-methoxypsoralen and UVA radiation, with re-infusion of photoactivated cells into the patient43. This induces DNA damage and apoptosis of exposed cells, with activated T lymphocytes preferentially affected44,45. As only 5-10% of lymphocytes are exposed during the procedure, which is insufficient to explain the effects of ECP, it is speculated that the apoptotic cells have indirect immunomodulatory actions on other immunocompetent cells43. These immunomodulatory mechanisms are poorly understood, but generation of regulatory T lymphocytes (Tregs), alteration of cytokine patterns, and modulation of dendritic cells (DCs) appear to be fundamental46–52.
The modulation of DCs includes increased number due to differentiation of ECP-exposed monocytes53,54 and stimulation of a DC-tolerogenic state upon phagocytosis of apoptosed cells, characterised by down-regulation of maturation markers and co-stimulatory molecules and increased secretion of anti-inflammatory cytokines, particularly interleukin-1055–60. Upon interaction with T lymphocytes, tolerogenic DCs can induce T lymphocyte anergy or apoptosis or stimulate Treg production58,61. In aGvHD, DCs, as the major APC, present disparate host antigens to donor T lymphocytes, propagating the pathway of cellular injury. Inducing a DC-tolerogenic state and dampening T lymphocyte activation could attenuate the trigger for aGvHD. The modulation of DC number and function may be a central mechanism of ECP. Tregs are essential in maintaining self-tolerance, down-regulating immune responses, and limiting inflammation that may be harmful to the host and contribute to the mechanism of ECP62–67.
The unique advantage of ECP as a therapy is lack of global immunosuppression but preservation of the graft-versus-leukaemia effect68. Promoting immune tolerance, with selective down-regulation of immune stimulation, could reduce aGvHD and enable a reduction in other immunosuppression, facilitating thymic recovery, restoration of normal T lymphopoiesis, and complete IR (Figure 1) with improved clinical outcome as ability to fight infections improves and risk of secondary malignancy or relapse diminishes. It is well tolerated with few adverse effects, and reports of clinical efficacy are impressive69–77. Whilst the immune-sparing effects of ECP have been demonstrated78,79, further randomised controlled studies are needed as well as investigation of the effect of ECP on thymic recovery. Further elucidation of the underlying mechanisms at play, as well as the optimal treatment schedule, is required to ascertain fully the role of ECP in aGvHD treatment.
Abbreviations
aGvHD, acute graft-versus-host disease; APC, antigen-presenting cell; CDR3, complementarity determining region 3; DC, dendritic cell; ECP, extracorporeal photopheresis; HSCT, allogeneic hematopoietic stem cell transplant; IFNγ, interferon gamma; IR, immunoreconstitution; MHC, major histocompatibility complex; mTOR, mammalian target of rapamycin; NK, natural killer; STAT1, signal transducer and activator of transcription 1; TCR, T cell receptor; TEC, thymic epithelial cell; TREC, T cell receptor excision circle; Treg, regulatory T lymphocyte.
Competing interests
The author(s) declared that they have no competing interests.
Grant information
Aisling Flinn is funded by the Bubble Foundation UK.
Faculty Opinions recommendedReferences
- 1.
Ljungman P, Bregni M, Brune M, et al.:
Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009.
Bone Marrow Transplant.
2010; 45(2): 219–34. PubMed Abstract
| Publisher Full Text
- 2.
Burroughs L, Woolfrey A:
Hematopoietic cell transplantation for treatment of primary immune deficiencies.
Cell Ther Transplant.
2010; 2(8): PubMed Abstract
| Publisher Full Text
| Free Full Text
- 3.
Bosch M, Khan FM, Storek J:
Immune reconstitution after hematopoietic cell transplantation.
Curr Opin Hematol.
2012; 19(4): 324–35. PubMed Abstract
| Publisher Full Text
- 4.
Antin JH:
Immune reconstitution: the major barrier to successful stem cell transplantation.
Biol Blood Marrow Transplant.
2005; 11(2 Suppl 2): 43–5. PubMed Abstract
| Publisher Full Text
- 5.
Krenger W, Holländer GA:
The role of the thymus in allogeneic hematopoietic stem cell transplantation.
Swiss Med Wkly.
2010; 140: w13051. PubMed Abstract
| Publisher Full Text
- 6.
Storek J, Geddes M, Khan F, et al.:
Reconstitution of the immune system after hematopoietic stem cell transplantation in humans.
Semin Immunopathol.
2008; 30(4): 425–37. PubMed Abstract
| Publisher Full Text
- 7.
Krenger W, Blazar BR, Holländer GA:
Thymic T-cell development in allogeneic stem cell transplantation.
Blood.
2011; 117(25): 6768–76. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 8.
Dumont-Girard F, Roux E, van Lier RA, et al.:
Reconstitution of the T-cell compartment after bone marrow transplantation: restoration of the repertoire by thymic emigrants.
Blood.
1998; 92(11): 4464–71. PubMed Abstract
- 9.
Hauri-Hohl MM, Keller MP, Gill J, et al.:
Donor T-cell alloreactivity against host thymic epithelium limits T-cell development after bone marrow transplantation.
Blood.
2007; 109(9): 4080–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 10.
Clave E, Busson M, Douay C, et al.:
Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation.
Blood.
2009; 113(25): 6477–84. PubMed Abstract
| Publisher Full Text
- 11.
Toubert A, Glauzy S, Douay C, et al.:
Thymus and immune reconstitution after allogeneic hematopoietic stem cell transplantation in humans: never say never again.
Tissue Antigens.
2012; 79(2): 83–9. PubMed Abstract
| Publisher Full Text
- 12.
Langerak AW, van Den Beemd R, Wolvers-Tettero IL, et al.:
Molecular and flow cytometric analysis of the Vbeta repertoire for clonality assessment in mature TCRalphabeta T-cell proliferations.
Blood.
2001; 98(1): 165–73. PubMed Abstract
| Publisher Full Text
- 13.
Memon SA, Sportés C, Flomerfelt FA, et al.:
Quantitative analysis of T cell receptor diversity in clinical samples of human peripheral blood.
J Immunol Methods.
2012; 375(1–2): 84–92. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 14.
Genevée C, Diu A, Nierat J, et al.:
An experimentally validated panel of subfamily-specific oligonucleotide primers (V alpha 1-w29/V beta 1-w24) for the study of human T cell receptor variable V gene segment usage by polymerase chain reaction.
Eur J Immunol.
1992; 22(5): 1261–9. PubMed Abstract
| Publisher Full Text
- 15.
Six A, Mariotti-Ferrandiz ME, Chaara W, et al.:
The past, present, and future of immune repertoire biology - the rise of next-generation repertoire analysis.
Front Immunol.
2013; 4: 413. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 16.
Hazenberg MD, Otto SA, de Pauw ES, et al.:
T-cell receptor excision circle and T-cell dynamics after allogeneic stem cell transplantation are related to clinical events.
Blood.
2002; 99(9): 3449–53. PubMed Abstract
| Publisher Full Text
- 17.
Clave E, Lisini D, Douay C, et al.:
A low thymic function is associated with leukemia relapse in children given T-cell-depleted HLA-haploidentical stem cell transplantation.
Leukemia.
2012; 26(8): 1886–8. PubMed Abstract
| Publisher Full Text
- 18.
Olkinuora H, Talvensaari K, Kaartinen T, et al.:
T cell regeneration in pediatric allogeneic stem cell transplantation.
Bone Marrow Transplant.
2007; 39(3): 149–56. PubMed Abstract
| Publisher Full Text
- 19.
Welniak LA, Blazar BR, Murphy WJ:
Immunobiology of allogeneic hematopoietic stem cell transplantation.
Annu Rev Immunol.
2007; 25: 139–70. PubMed Abstract
| Publisher Full Text
- 20.
Ferrara JL, Levine JE, Reddy P, et al.:
Graft-versus-host disease.
Lancet.
2009; 373(9674): 1550–61. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 21.
Sung AD, Chao NJ:
Concise review: acute graft-versus-host disease: immunobiology, prevention, and treatment.
Stem Cells Transl Med.
2013; 2(1): 25–32. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 22.
Krenger W, Rossi S, Piali L, et al.:
Thymic atrophy in murine acute graft-versus-host disease is effected by impaired cell cycle progression of host pro-T and pre-T cells.
Blood.
2000; 96(1): 347–54. PubMed Abstract
- 23.
Krenger W, Holländer GA:
The immunopathology of thymic GVHD.
Semin Immunopathol.
2008; 30(4): 439–56. PubMed Abstract
| Publisher Full Text
- 24.
Seemayer TA, Lapp WS, Bolande RP:
Thymic involution in murine graft-versus-host reaction. Epithelial injury mimicking human thymic dysplasia.
Am J Pathol.
1977; 88(1): 119–34. PubMed Abstract
| Free Full Text
- 25.
Krenger W, Rossi S, Holländer GA:
Apoptosis of thymocytes during acute graft-versus-host disease is independent of glucocorticoids.
Transplantation.
2000; 69(10): 2190–3. PubMed Abstract
- 26.
Krenger W, Holländer GA:
The thymus in GVHD pathophysiology.
Best Pract Res Clin Haematol.
2008; 21(2): 119–28. PubMed Abstract
| Publisher Full Text
- 27.
Weinberg K, Blazar BR, Wagner JE, et al.:
Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation.
Blood.
2001; 97(5): 1458–66. PubMed Abstract
| Publisher Full Text
- 28.
Dulude G, Roy DC, Perreault C:
The effect of graft-versus-host disease on T cell production and homeostasis.
J Exp Med.
1999; 189(8): 1329–42. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 29.
Holländer GA, Widmer B, Burakoff SJ:
Loss of normal thymic repertoire selection and persistence of autoreactive T cells in graft vs host disease.
J Immunol.
1994; 152(4): 1609–17. PubMed Abstract
- 30.
Tivol E, Komorowski R, Drobyski WR:
Emergent autoimmunity in graft-versus-host disease.
Blood.
2005; 105(12): 4885–91. PubMed Abstract
| Publisher Full Text
- 31.
Teshima T, Reddy P, Liu C, et al.:
Impaired thymic negative selection causes autoimmune graft-versus-host disease.
Blood.
2003; 102(2): 429–35. PubMed Abstract
| Publisher Full Text
- 32.
Sakoda Y, Hashimoto D, Asakura S, et al.:
Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease.
Blood.
2007; 109(4): 1756–64. PubMed Abstract
| Publisher Full Text
- 33.
MacMillan ML, Weisdorf DJ, Wagner JE, et al.:
Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems.
Biol Blood Marrow Transplant.
2002; 8(7): 387–94. PubMed Abstract
| Publisher Full Text
- 34.
Kong FK, Chen CL, Cooper MD:
Reversible disruption of thymic function by steroid treatment.
J Immunol.
2002; 168(12): 6500–5. PubMed Abstract
| Publisher Full Text
- 35.
Martin PJ, Rizzo JD, Wingard JR, et al.:
First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation.
Biol Blood Marrow Transplant.
2012; 18(8): 1150–63. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 36.
Dignan FL, Clark A, Amrolia P, et al.:
Diagnosis and management of acute graft-versus-host disease.
Br J Haematol.
2012; 158(1): 30–45. PubMed Abstract
| Publisher Full Text
- 37.
Rizk M, Monaghan M, Shorr R, et al.:
Heterogeneity in Studies of Mesenchymal Stromal Cells to Treat or Prevent Graft-versus-Host Disease: A Scoping Review of the Evidence.
Biol Blood Marrow Transplant.
2016; pii: S1083-8791(16)30029-5. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 38.
Kim N, Im KI, Lim JY, et al.:
Mesenchymal stem cells for the treatment and prevention of graft-versus-host disease: experiments and practice.
Ann Hematol.
2013; 92(10): 1295–308. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 39.
Prasad VK, Lucas KG, Kleiner GI, et al.:
Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal™) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study.
Biol Blood Marrow Transplant.
2011; 17(4): 534–41. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 40.
Seggewiss R, Einsele H:
Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update.
Blood.
2010; 115(19): 3861–8. PubMed Abstract
| Publisher Full Text
- 41.
Fry TJ, Mackall CL:
Immune reconstitution following hematopoietic progenitor cell transplantation: challenges for the future.
Bone Marrow Transplant.
2005; 35(Suppl 1): S53–7. PubMed Abstract
| Publisher Full Text
- 42.
Kitko CL, Levine JE:
Extracorporeal photopheresis in prevention and treatment of acute GVHD.
Transfus Apher Sci.
2015; 52(2): 151–6. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 43.
Knobler R, Barr ML, Couriel DR, et al.:
Extracorporeal photopheresis: past, present, and future.
J Am Acad Dermatol.
2009; 61(4): 652–65. PubMed Abstract
| Publisher Full Text
- 44.
Hannani D, Merlin E, Gabert F, et al.:
Photochemotherapy induces a faster apoptosis of alloreactive activated T cells than of nonalloreactive resting T cells in graft versus host disease.
Transplantation.
2010; 90(11): 1232–8. PubMed Abstract
| Publisher Full Text
- 45.
Tambur AR, Ortegel JW, Morales A, et al.:
Extracorporeal photopheresis induces lymphocyte but not monocyte apoptosis.
Transplant Proc.
2000; 32(4): 747–8. PubMed Abstract
| Publisher Full Text
- 46.
Xia CQ, Campbell KA, Clare-Salzler MJ:
Extracorporeal photopheresis-induced immune tolerance: a focus on modulation of antigen-presenting cells and induction of regulatory T cells by apoptotic cells.
Curr Opin Organ Transplant.
2009; 14(4): 338–43. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 47.
Gorgun G, Miller KB, Foss FM:
Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease.
Blood.
2002; 100(3): 941–7. PubMed Abstract
| Publisher Full Text
- 48.
Bladon J, Taylor PC:
Extracorporeal photopheresis: a focus on apoptosis and cytokines.
J Dermatol Sci.
2006; 43(2): 85–94. PubMed Abstract
| Publisher Full Text
- 49.
George JF, Gooden CW, Guo L, et al.:
Role for CD4+CD25+ T cells in inhibition of graft rejection by extracorporeal photopheresis.
J Heart Lung Transplant.
2008; 27(6): 616–22. PubMed Abstract
| Publisher Full Text
- 50.
Di Renzo M, Rubegni P, Pasqui AL, et al.:
Extracorporeal photopheresis affects interleukin (IL)-10 and IL-12 production by monocytes in patients with chronic graft-versus-host disease.
Br J Dermatol.
2005; 153(1): 59–65. PubMed Abstract
| Publisher Full Text
- 51.
Dieterlen MT, Bittner HB, Pierzchalski A, et al.:
Immunological monitoring of extracorporeal photopheresis after heart transplantation.
Clin Exp Immunol.
2014; 176(1): 120–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 52.
Heshmati F:
Updating ECP action mechanisms.
Transfus Apher Sci.
2014; 50(3): 330–9. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 53.
Berger C, Hoffmann K, Vasquez JG, et al.:
Rapid generation of maturationally synchronized human dendritic cells: contribution to the clinical efficacy of extracorporeal photochemotherapy.
Blood.
2010; 116(23): 4838–47. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 54.
Edelson RL:
Mechanistic insights into extracorporeal photochemotherapy: efficient induction of monocyte-to-dendritic cell maturation.
Transfus Apher Sci.
2014; 50(3): 322–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 55.
Holtick U, Marshall SR, Wang XN, et al.:
Impact of psoralen/UVA-treatment on survival, activation, and immunostimulatory capacity of monocyte-derived dendritic cells.
Transplantation.
2008; 85(5): 757–66. PubMed Abstract
| Publisher Full Text
- 56.
Spisek R, Gasova Z, Bartunkova J:
Maturation state of dendritic cells during the extracorporeal photopheresis and its relevance for the treatment of chronic graft-versus-host disease.
Transfusion.
2006; 46(1): 55–65. PubMed Abstract
| Publisher Full Text
- 57.
Di Renzo M, Sbano P, De Aloe G, et al.:
Extracorporeal photopheresis affects co-stimulatory molecule expression and interleukin-10 production by dendritic cells in graft-versus-host disease patients.
Clin Exp Immunol.
2008; 151(3): 407–13. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 58.
Capitini CM, Davis JP, Larabee SM, et al.:
Extracorporeal photopheresis attenuates murine graft-versus-host disease via bone marrow-derived interleukin-10 and preserves responses to dendritic cell vaccination.
Biol Blood Marrow Transplant.
2011; 17(6): 790–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 59.
Futterleib JS, Feng H, Tigelaar RE, et al.:
Activation of GILZ gene by photoactivated 8-methoxypsoralen: potential role of immunoregulatory dendritic cells in extracorporeal photochemotherapy.
Transfus Apher Sci.
2014; 50(3): 379–87. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 60.
Legitimo A, Consolini R, Failli A, et al.:
In vitro treatment of monocytes with 8-methoxypsolaren and ultraviolet A light induces dendritic cells with a tolerogenic phenotype.
Clin Exp Immunol.
2007; 148(3): 564–72. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 61.
Morelli AE, Thomson AW:
Tolerogenic dendritic cells and the quest for transplant tolerance.
Nat Rev Immunol.
2007; 7(8): 610–21. PubMed Abstract
| Publisher Full Text
- 62.
Gatza E, Rogers CE, Clouthier SG, et al.:
Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells.
Blood.
2008; 112(4): 1515–21. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 63.
Lamioni A, Parisi F, Isacchi G, et al.:
The immunological effects of extracorporeal photopheresis unraveled: induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo.
Transplantation.
2005; 79(7): 846–50. PubMed Abstract
| Publisher Full Text
- 64.
Biagi E, Di Biaso I, Leoni V, et al.:
Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+ functional regulatory T-cells in patients with graft-versus-host disease.
Transplantation.
2007; 84(1): 31–9. PubMed Abstract
| Publisher Full Text
- 65.
Lorenz K, Rommel K, Mani J, et al.:
Modulation of lymphocyte subpopulations by extracorporeal photopheresis in patients with acute graft-versus-host disease or graft rejection.
Leuk Lymphoma.
2015; 56(3): 671–5. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 66.
Maeda A, Schwarz A, Kernebeck K, et al.:
Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells.
J Immunol.
2005; 174(10): 5968–76. PubMed Abstract
| Publisher Full Text
- 67.
Schmitt S, Johnson TS, Karakhanova S, et al.:
Extracorporeal photophoresis augments function of CD4+CD25+FoxP3+ regulatory T cells by triggering adenosine production.
Transplantation.
2009; 88(3): 411–6. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 68.
Marshall SR:
Technology insight: ECP for the treatment of GvHD--can we offer selective immune control without generalized immunosuppression?
Nat Clin Pract Oncol.
2006; 3(6): 302–14. PubMed Abstract
| Publisher Full Text
- 69.
Calore E, Marson P, Pillon M, et al.:
Treatment of Acute Graft-versus-Host Disease in Childhood with Extracorporeal Photochemotherapy/Photopheresis: The Padova Experience.
Biol Blood Marrow Transplant.
2015; 21(11): 1963–72. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 70.
Bruserud Ø, Tvedt TH, Paulsen PQ, et al.:
Extracorporeal photopheresis (photochemotherapy) in the treatment of acute and chronic graft versus host disease: immunological mechanisms and the results from clinical studies.
Cancer Immunol Immunother.
2014; 63(8): 757–77. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 71.
Heshmati F:
Extra corporeal photo chemotherapy (ECP) in acute and chronic GVHD.
Transfus Apher Sci.
2010; 43(2): 211–5. PubMed Abstract
| Publisher Full Text
- 72.
Das-Gupta E, Dignan F, Shaw B, et al.:
Extracorporeal photopheresis for treatment of adults and children with acute GVHD: UK consensus statement and review of published literature.
Bone Marrow Transplant.
2014; 49(10): 1251–8. PubMed Abstract
| Publisher Full Text
- 73.
Rutella S, Valentini CG, Ceccarelli S, et al.:
Extracorporeal photopheresis for paediatric patients experiencing graft-versus-host disease (GVHD).
Transfus Apher Sci.
2014; 50(3): 340–8. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 74.
Abu-Dalle I, Reljic T, Nishihori T, et al.:
Extracorporeal photopheresis in steroid-refractory acute or chronic graft-versus-host disease: results of a systematic review of prospective studies.
Biol Blood Marrow Transplant.
2014; 20(11): 1677–86. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 75.
Messina C, Locatelli F, Lanino E, et al.:
Extracorporeal photochemotherapy for paediatric patients with graft-versus-host disease after haematopoietic stem cell transplantation.
Br J Haematol.
2003; 122(1): 118–27. PubMed Abstract
| Publisher Full Text
- 76.
Berger M, Pessolano R, Albiani R, et al.:
Extracorporeal photopheresis for steroid resistant graft versus host disease in pediatric patients: a pilot single institution report.
J Pediatr Hematol Oncol.
2007; 29(10): 678–87. PubMed Abstract
| Publisher Full Text
- 77.
Hart JW, Shiue LH, Shpall EJ, et al.:
Extracorporeal photopheresis in the treatment of graft-versus-host disease: evidence and opinion.
Ther Adv Hematol.
2013; 4(5): 320–34. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 78.
Ussowicz M, Musial J, Mielcarek M, et al.:
Steroid-sparing effect of extracorporeal photopheresis in the therapy of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation.
Transplant Proc.
2013; 45(9): 3375–80. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 79.
Dignan FL, Aguilar S, Scarisbrick JJ, et al.:
Impact of extracorporeal photopheresis on skin scores and quality of life in patients with steroid-refractory chronic GVHD.
Bone Marrow Transplant.
2014; 49(5): 704–8. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation

Comments on this article Comments (0)