Keywords
Tumor sampling, cutting grid, divide and conquer, clear cell renal cell carcinoma, intratumor heterogeneity, pathology routine
Tumor sampling, cutting grid, divide and conquer, clear cell renal cell carcinoma, intratumor heterogeneity, pathology routine
In this second version, as suggested by reviewers, we have provided more technical details of the DAC implementation. In particular, we added two new paragraphs in Methods and Results and another one in Discussion.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
In the light of current findings provided by numerous sequencing tools, it is known that practically all human neoplasms display some degree of intratumor heterogeneity (ITH)1. Characteristically, ITH is not uniformly distributed along the tumor; instead, it shows a regional distribution following a stochastic pattern, the final result being unique, unpredictable, and dynamically varying along the time2. The correct identification of ITH is mandatory now that targeted therapies are offering promising results to patients3, but pathologists - the specialists in charge of tumor selection for analysis - seem to have not given, so far, an appropriate answer to this issue.
We have recently proposed a reliable, affordable and time-saving solution to this problem4. The goal is twofold; to improve ITH detection and to perform ITH at affordable laboratory costs. This simple solution is based on the divide-and-conquer algorithm (DAC). Noteworthy, tumor sampling following DAC outperforms the routine protocol sampling for identifying ITH and, it does it, at a similar cost4. However, pathologists could consider that DAC is a time-consuming method when grossing, which might make it difficult to introduce it in routine practice. In this brief report, we describe a simple procedure to overcome this problem.
The so-called DAC algorithm5 is based on recursively breaking down a problem in smaller parts (divide) until these are simple enough to be solved directly (conquer). Then, partial solutions are combined to solve the original problem. DAC strategies have been largely applied in science to solve complex problems, including several challenging issues in biomedical areas. As early as in 1967, DAC helped clinicians to correlate hypoglycemia with infantile convulsions6. In addition, DAC has been useful in cell biology and oncology, for instance in selecting the appropriate cells for biological experiments7 and, more recently, in helping to decipher breast cancer heterogeneity8.
Here DAC is applied to clear cell renal cell carcinomas (ccRCCs), since these tumors are frequently large and, for this reason, impossible to be totally sampled. Any other large tumor, however, can benefit from this method. The DAC strategy (Figure 1) requires the pathologist to select, instead of a few large fragments, a substantial number of small ones widely distributed along the entire tumor. However, pathologists under a daily routine pressure can perceive this method as laborious and time-consuming.
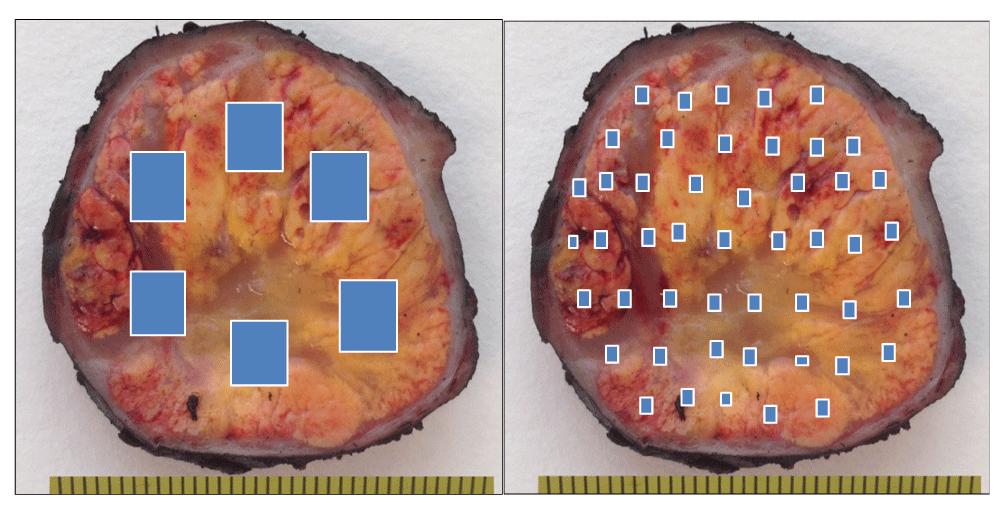
A simple device consisting of a cutting grid (here, a potato cutter) will overcome this inconvenience. When applied directly to the whole tumor surface previously thin-sliced, the grid will cut it into small cubes in one shot (Figure 2). Next, the pathologist’s decision will consist simply in selecting the cubes that will be processed for analysis as previously reported4. The method can be applied (and has been tested) to both fresh and formalin-fixed tissue, saves time, and assures a uniform sampling distribution along the tumor. The objective for improving efficiency of targeted therapies is the discovery of the complete ITH spectrum, and not its exact location. Thus the selected cubes included in the cassettes (six to eight cubes per cassette) will provide much more thorough information of the tumor, both under the microscope as well as at the molecular level.
A big tumor size is not a limitation. If the tumor is bigger than the cutting grid, the pathologist may apply the DAC strategy and subdivide the whole slice into smaller parts before applying the cutting device to each one of these portions. Since the final goal of this strategy is a thorough tumor sampling, the slicing in the grossing room does not need to be necessarily perfect. Obtaining a whole and homogeneous slice in a huge tumor, in a tumor with heterogeneous consistency, or in a tumor with multifocal necrosis, may be a difficult task under daily routine pressure. For these cases, partial thin slices of the whole tumor circumference might be also appropriate for this sampling strategy. In fact, it is a more advantageous and time-saving attitude to obtain multiple thin (with thickness smaller than 3 mm) sectorial slices along the tumor than complete irregular thicker (bigger than 3 mm) slices, as the cubes obtained by the subsequent application of the cutting grid will not need to be additionally thinned one by one with a scalpel to fit them into the cassette.
We have designed the DAC strategy to enhance ITH detection in big tumors at the same cost4. For this reason, we propose to respect what the international protocols advice, that is, one cassette per centimeter of tumor diameter. Extreme cases will follow the same rule. Thus, a 20 cm in diameter renal tumor would generate 20 cassettes harboring in total 120 to 160 tumor samples (six to eight per cassette) thus assuring a thorough sampling. As usual, the decision of which are the cubes to be selected in the sampling is a pathologist's personal decision. The common sense says that any area with different consistency or color should be sampled, but if the tumor is totally homogeneous by naked eye we propose a balanced geographical selection of cubes in each tumor slice. Additionally, a small fragment of normal renal tissue can be included in some cassettes to have an internal control for eventual immunohistochemical or molecular studies.
The use of the DAC method to help sampling strategies is not new. Indeed some authors have applied the algorithm in particle physics to improve the diffusion sampling in generalized ensemble simulations9. This approach, or any other with scientific basis, has not been implemented for tissue selection in Pathology laboratories so far, since the pathologists did not consider tumor sampling a complex problem in the pre-molecular era.
An experience-based reasoning says that this option will save pathologist’s time when handling large tumors, in a manner which is inexpensive and reliable at the same time. In combination with the changes proposed for the technician training in our previous report4, this new alternative will make the pathologists’ routine much faster and robust providing an integrated solution to fulfill basic researchers’ expectations10. If the DAC strategy is adopted as a suitable method to increase the amount of information given to oncologists, pathologist's routine will move from the classic big-fragments-into-the-cassette routine to a sort of rudimentary tissue microarray building, as recently proposed4.
Figures are demonstrative. For instance, the DAC strategy applied to a ccRCC of 10 cm in diameter - a quite common situation in routine pathology - will generate approximately 80 small samples (of about 4–5 mm in size) that would be included in 10 cassettes for a thorough tumor examination. Importantly to remark, the same 10 cm in diameter tumor would need also 10 cassettes for the analysis, with one tumor sample per cassette, in the case of routine sampling protocols11.
Depending on the pathologist’s skills, the time to collect 80 small samples in the grossing room is variable, but in any case, long. For this reason, any successful alternative must necessarily overcome this hurdle. A feasible choice would be an electric bacon slicer, but a long bladed knife will also work. To note, slicing electric machines are being increasingly used in pathology for handling radical prostatectomies12 and other surgical specimens13, and they are the first step in the whole-mounting processing for tumor mapping. In this case, the obtained ccRCC slices can be quickly cut in one shot by pressing on the entire tumor surface with a cutting grid. The procedure will generate many cubes ready to be included within a cassette. If we assume that tumor sampling following a DAC strategy is appropriate for improving ITH detection, the use of a cutting grid will shorten significantly the total process ensuring a uniform and widespread selection of the samples. A straightforward estimation with some practical cases indicates that the time for obtaining 80 samples with this method is reduced to a tenth as we cut 10 small tumor pieces at the same time.
DAC strategy, by definition, is not necessary in small tumors if they can be totally sampled. It is the policy of any Laboratory to fix the maximum tumor size for performing a total tumor sampling, since there are no precise recommendations in the international protocols. In this setting, the final decision will probably depend on the routine pressure, local customs and habits and, more important, on the varying annual budget assigned to the Laboratory of Pathology.
The present paper describes a new method for tumor sampling in routine pathology inspired by the DAC algorithm4. Once DAC has been proved to be efficient for ITH detection, we expect that the use of a cutting grid will make affordable its widespread application. Objectives are twofold: ITH detection improvement and time optimization (cost) in Pathology laboratories.
JIL and JMC identified the problem and gave a realistic solution. JIL and JMC wrote this note.
JMC is funded by Ikerbasque: The Basque Foundation for Science.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 26 Jul 16 |
read | read | |
|
Version 1 06 Jul 16 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)