Keywords
FASD, Fetal Alcohol Exposure, ethanol, chronic, outbred, Swiss-Webster mice, motor skills
FASD, Fetal Alcohol Exposure, ethanol, chronic, outbred, Swiss-Webster mice, motor skills
Ethanol (EtOH) is a toxin that produces variable detrimental physical and neurobehavioral effects in individuals subjected to ethanol exposure in utero, possibly resulting in Fetal Alcohol Spectrum Disorder (FASD). Animal models can replicate the human condition of FASD relatively well (Wilson & Cudd, 2011), but the broad spectrum of human drinking choices made during pregnancy is reflected in the enormous number of different FASD models currently employed by researchers. Even within a single species, differences in acute versus chronic exposure, the timing of the exposure, route of administration, and forced versus voluntary drinking patterns can result in a vast array of possible phenotypes.
The use of inbred mice has decreased variability in a field with a vast variety of models and made it easier to compare data across paradigms, explore voluntary drinking paradigms, and identify specific genetic differences regulating individual sensitivity to ethanol (Mayfield et al., 2008). Genetic variation causes some strains of mice to voluntarily drink more than other strains (Rhodes et al., 2007) — For example, C57BL/6 mice are heavy drinkers, while DBA2J mice are abstainers (Rhodes et al., 2005). Many FASD studies currently use inbred C57B/6 mice because of the reliability of consumption in this strain (Rhodes et al., 2005).
Inbred mouse strains exposed to chronic gestational ethanol have demonstrated deficits in motor function and learning/memory (Brady et al., 2012; Sanchez Vega et al., 2013). Typically, motor reflexes/coordination and learning/memory skills appear to be less dependent on the timing of alcohol exposure than activity and anxiety-related behavioral changes, which have more narrow windows (Mantha et al., 2013).
However, use of inbred strains do not faithfully replicate the genetic variation that naturally occurs within the human condition, so outbred strains also provide valuable information about FASD. Outbred Wistar rats are commonly used as a FASD rodent model, but use of outbred mouse models is much less common. Previously reported inbred alcohol-induced deficits may not show up in an outbred mouse model—for example, corticothalamic differences were not observed in an outbred mouse FASD model (White et al., 2015). But a recent paper characterized the effect of moderate prenatal ethanol exposure on outbred Swiss Webster mouse neonate behavior (including surface righting, negative geotaxis, cliff aversion, auditory startle, ear twitch, open field traversal, air righting, and eye opening) and found that ethanol-exposed pups achieved some developmental milestones (surface righting, cliff aversion, and open field traversal) at a different rate than non-exposed control pups (Chi et al., 2016).
To examine whether observed deficits in outbred mouse neonates can persist into adulthood, we examined the effect of chronic moderate gestational ethanol exposure on adult neurobehavioral outcomes in the offspring previously studied by Chi & colleagues (2016). We measured both learning/memory and motor skill subsets in male mice in order to better understand the behavioral impact of developmental ethanol exposure in an outbred model.
All experimentation was compliant with the Hampden-Sydney Institutional Animal Care and Use Committee (IACUC). Twenty-four outbred male Swiss Webster mice were obtained from Ursinus College (four litters per treatment of two-four male mice randomly selected from each litter; 24 animals in total) following completion of the early postnatal testing (Chi et al., 2016). All of the dams in the ethanol treatment group were found to have moderate gestational exposure after using the Drinking in the Dark (Boehm et al., 2008) paradigm, consuming an average of 4.41 g/kg ± .43 standard error (SE) (Table 1). All mice were male and group housed by litter, with a 12-hour light cycle and food and water ad libitum. The weights of the mice at 6 months were not significantly different (by t-test, p=0.43795; mean EtOH=39.16g, SE=0.68; mean H2O=39.46g, SE 1.64). All behavioral tests were performed between 4–6 months of age in the following order to reduce the impact that exposure to testing conditions might have on subsequent testing performance: open field, grip strength, Barnes maze, rotarod (a rotating rod). For each measure, scores for all littermates were averaged together before statistical analysis (12 animals in four litters per treatment group) to account for the variability in gestational ethanol exposure that accompanies a voluntary drinking paradigm.
The open field test was performed using an opaque box constructed of 6 mm plexiglass (56 cm × 56 cm × 51 cm) as a measure of anxiety-like behavior and locomotion. Testing was modeled after Nunes & colleagues (2011). The first 2 minutes of data were analyzed and the field was divided into nine equal sections. Total squares traveled and time spent in the center square were analyzed for relevance using unpaired t-tests.
The Barnes maze was used to test for cognitive deficits in learning and memory. Litters were brought into the testing room one at a time to run the Barnes maze using a modified version of the shortened paradigm outlined by Attar et al. (2013) with a 120w light source suspended 1 meter above the stage in the absence of a noxious noise. During habituation and trial days, mice were allowed 30 seconds to enter into the target hole voluntarily before they were coaxed in. In addition, the escape cage remained on the probe day. Entrance into the correct hole, latency to enter, and the percent of holes explored in the opposite quadrant were assessed, as well as the number of holes explored before and after finding the correct hole. Probe day data were analyzed using unpaired t-tests.
The Kondziela Inverted Screen test was modeled after Deacon (2013) and measures the overall strength of the mice, as well as their ability to maintain their equilibrium while inverted.
The latency of the mice to fall was tested and an average of their three recordings was taken with an upper limit of 10 minutes per trial. After each trial, the mice were returned to their home cages and allowed to rest approximately 1 hour until the sequential order called for the next test. The mice were randomly selected for the first trial, but the order was maintained for the two subsequent trials. Average latency to fall was analyzed using unpaired t-tests.
The rotarod was chosen to test both motor learning and endurance. The rotarod (San Diego Instruments) is a spinning rod with dividers separating individual testing spaces. It can be set to turn at a certain rotation per minute (RPM) and to increase RPM at a determined rate. When the mice fall off, sensors on the floor stop the clock and record the number of seconds and the precise RPM.
Mice were daily trained on the rotarod over a 3-day period, rested for 1 day, and then exposed to a performance day. All 24 mice were trained to run on the rotarod in a similar manner as Gill & Dietrich (1998) with modifications. Each mouse had a single daily training trial on each of three consecutive days that lasted until they fell off (up to 300 seconds) and animals were not placed on the rod again that day. Animals were rested for one day, followed by a performance assessment as outlined in Nozari et al. (2014). Mice started at zero RPM and accelerated by 6 RPM until the mice fell off. Each mouse ran the performance test three times and the average time per mouse was recorded.
The order that the mice were selected to run was randomized, but once that order was established, it remained unchanged. The latency to fall on the performance day and the percent improvement from the first day of training to the final performance day were separately analyzed using unpaired t-tests, and performance over the 3-day training period was assessed by a mixed ANOVA using SPSS.
In open field testing, the amount of time spent in the open center square was not significantly different between ethanol-exposed and control animals (by t-test, p=0.2383; mean EtOH=13.72, SE=8.535; mean H2O=6.973, SE=2.476; n=4 litters; Figure 1A). There was no significant difference in locomotor activity between ethanol-exposed and control mice, as measured by the mean number of squares traversed (by t-test, p=0.4231; mean EtOH=34.56, SE=9.691; mean H2O=37.83, SE=7.781; n=4; Figure 1B).
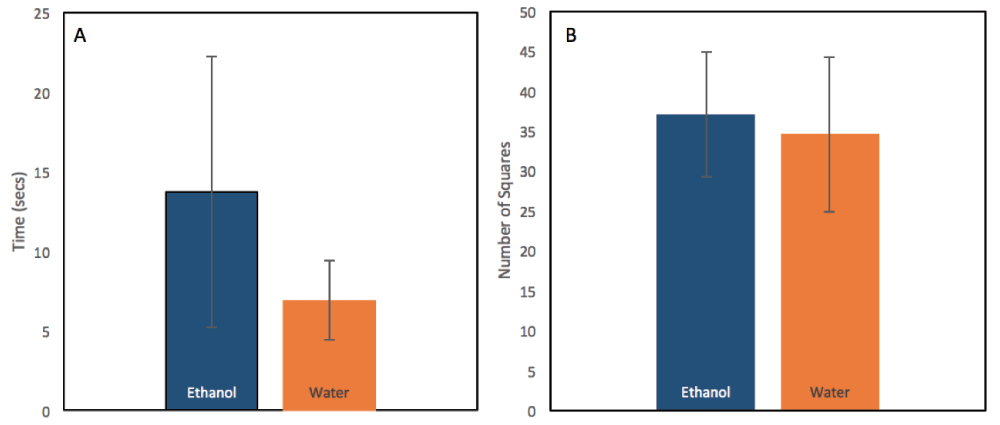
(A) There was no significant difference found between ethanol-exposed mice and control mice in the average number of seconds spent in the center square of the open field over a 2 minute period, as a measure of anxiety-like behavior (by t-test, p=0.2383; mean EtOH=13.72, SE=8.535; mean H2O=6.973, SE=2.476; n=4 litters). (B) No differences were found in the total number of squares crossed in the open field measure, as a measure of locomotion (by t-test, p=0.4231; mean EtOH=34.56, SE=9.691; mean H2O=37.83, SE=7.781).
Mice that were exposed to EtOH during development showed no differences on the probe day in total hole exploration (by t-test, p=0.7777; mean EtOH=6.96, SE=1.028; mean H2O=7.67, SE=2.12; n=4 litters), number of incorrect hole explorations before finding the target hole (by t-test, p=0.6009; mean EtOH=5.79, SE=0.3560; mean H2O=6.83, SE=1.766; n = 4), or the number of hole explorations after finding the target (by t-test, p=0.7352; mean EtOH=1.17, SE=0.7876; mean H2O=0.83, SE=0.50; n=4; Figure 2A). There was also no significant difference found in the percentage of holes explored on the opposite quadrant of the maze (by t-test, p=0.1272; mean EtOH=33.33, SE=9.129; mean H2O=57.50, SE=10.13; n= 4; Figure 2B).
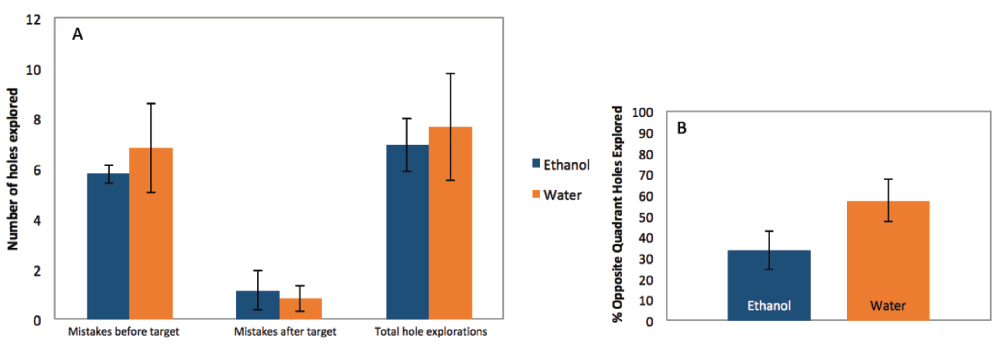
(A) There was no significant difference between ethanol-exposed mice and control mice in number of mistakes made before finding the target hole (by t-test, p=0.6009; mean EtOH=5.79, SE=0.3560; mean H2O=6.83, SE=1.766; n = 4 litters), the number of hole explorations after finding the target (by t-test, p=0.7352; mean EtOH=1.17, SE=0.7876; mean H2O=0.83, SE=0.50), or the total hole explorations on the final probe day (by t-test, p=0.7777; mean EtOH=6.96, SE=1.028; mean H2O=7.67, SE=2.12). (B) When the maze was divided into 4 quadrants for analysis, there was also no difference between the ethanol and control mice in the percent of holes located the opposite quadrant that were explored (by t-test, p=0.1272; mean EtOH=33.33, SE=9.129; mean H2O=57.50, SE=10.13).
There was no significant difference in grip strength between ethanol-exposed and control mice, as measured by the average latency to fall over the three trials (by t-test, p=0.855; mean EtOH=207.396 sec, SE=48.585; mean H2O=193.39, SE=41.221; n=4 litters; Figure 3).
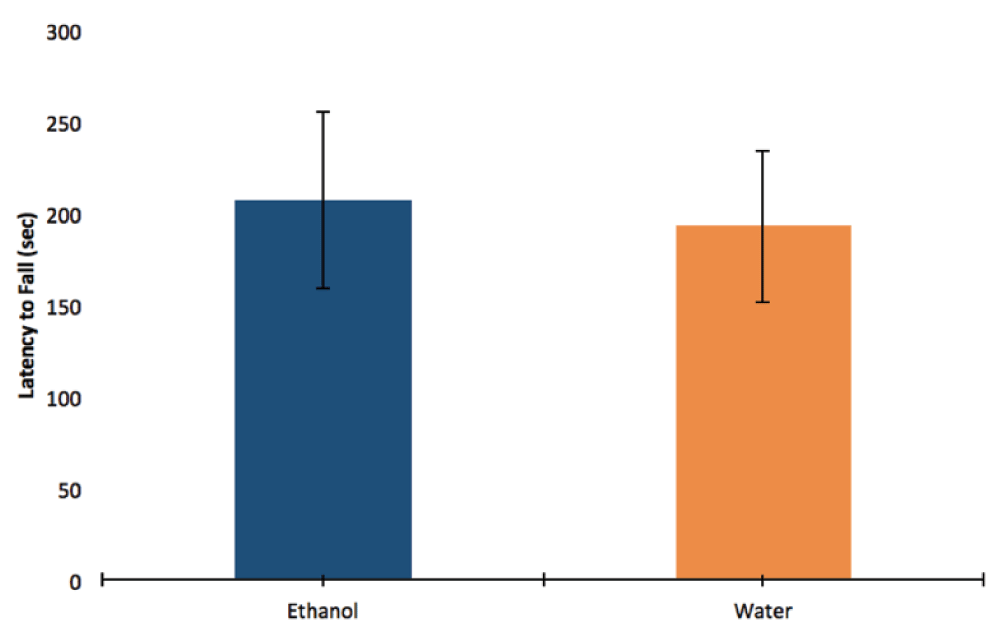
There was no significant difference found between ethanol-exposed mice and control mice in the latency to fall from the inverted screen as a measure of grip strength (by t-test, p=0.855; mean EtOH=207.396 seconds, SE=48.585; mean H2O=193.39, SE=41.221; n=4 litters).
By the final performance day, previous ethanol exposure had no effect on the max RPM that mice were able to achieve (by t-test, p=0.399; mean EtOH=7.19, SE=0.68; mean H2O=9.0, SE=1.78; n=4 litters; Figure 4A), indicating that overall motor performance after training was not affected by the gestational ethanol exposure. However, ethanol-exposed animals showed significantly less improvement between the first day of training and the final rotarod performance day, indicating a deficit in the acquisition of this motor skill (by t-test, p=0.008; mean EtOH=30.11% improvement, SE = 2.5%; mean H2O=53.52%, SE=5.5%; Figure 4B).
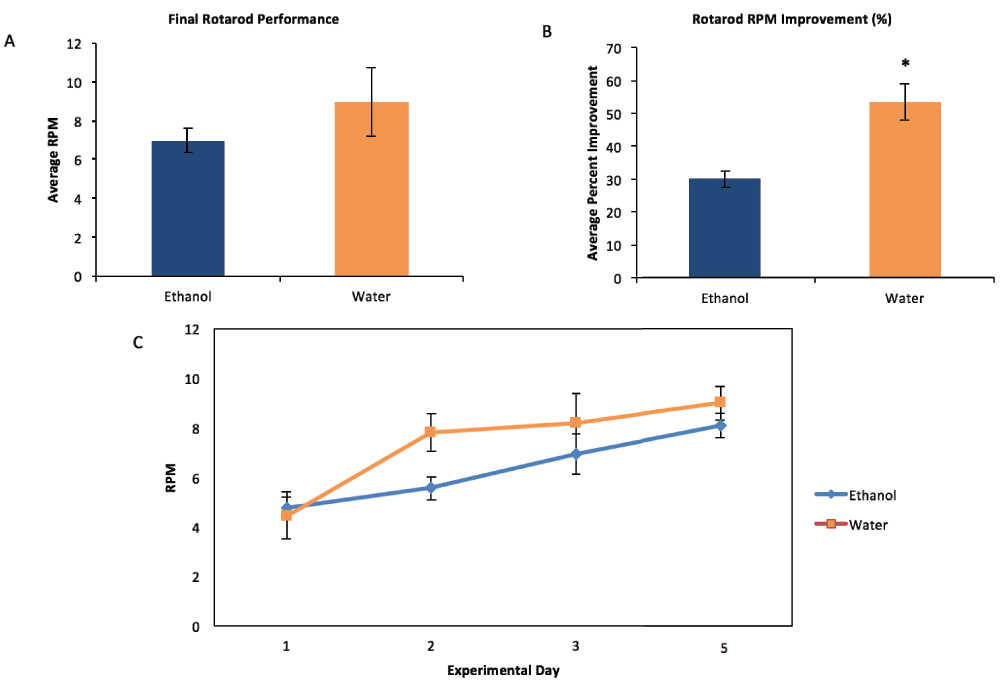
(A) There was no significant difference between ethanol-exposed mice and control mice in the average max RPM attained on the final performance day (5) (by t-test, p=0.399; mean EtOH=7.19, SE=0.68; mean H2O=9.0, SE=1.78; n=4 litters). (B) On average, control mice improved more than ethanol-exposed mice over the course of the rotarod testing, as measured by the percent change in RPM reached from the first training day (1) to the last performance day (5) (by t-test, p=0.008, EtOH mean=30.11%, SE=2.5%; H2O mean=53.52%, SE=5.5%). (C) We found a possible effect of gestational ethanol exposure on the max RPM at which the mice were able to run over the course of the three training days (F(2,12)=4.812, p=0.029), but this result was not significant after Sidak correction.
Both saline and ethanol treated mice showed an increase in the latency to fall in succeeding trial days at each age examined, indicating that both groups of mice were learning how to stay on the rod longer. Analysis by mixed ANOVA showed a significant effect of trial day over the three day training period [F(1,6)=37.694; p=0.01; Figure 4C]. We found a possible effect of gestational ethanol exposure on the max RPM at which the mice were able to run over the course of the three training days (F(2,12)=4.812, p=0.029; Figure 4C), but this result was not significant after Sidak correction.
Our experiment showed that chronic low/moderate gestational ethanol exposure had no effect on some motor measures in adult outbred mice, including grip strength and overall rotarod performance, yet mice exposed to prenatal ethanol did not improve as quickly when learning the rotarod activity. This adult rotarod learning deficits may be due to long-term ethanol induced changes in specific brain areas that are involved in the acquisition of complex motor skills.
A recent meta-analysis of human FASD concluded that prenatal ethanol exposure can result in gross motor deficits, including gait and balance problems (Lucas et al., 2014). Rodent studies have indicated that ethanol exposure, particularly in the third trimester equivalent, can impact cerebellar Purkinje cell development and alter motor behavior (Maier et al., 1999). The cerebellum is traditionally held responsible for the coordination and execution of motor skills, particularly gait and balance. Ethanol-induced cerebellar damage can cause FASD motor skill deficits, and compromised corticocerebellar circuits may result in impaired visuospatial abilities or other more cognitive processes. However, in rodent models, cerebellar Purkinje cells seem particularly vulnerable to degeneration when exposed to alcohol during the critical period that occurs during the early postnatal period (human third trimester equivalent) (Jaatinen & Rintala, 2008), whereas our mouse model was only exposed during the first and second trimester equivalents.
The striatum (caudate/putamen) also plays a central role in the acquisition of long term motor skills, and remains of particular interest to alcohol researchers because of its central role in addictive behavior. In rodents, moderate gestational ethanol exposure can alter medium spiny neuron dendritic branching in the striatum pups (Rice et al., 2012), and studies show that developmental ethanol exposure can alter brain-derived neurotrophic factor (BDNF) expression in adult offspring, which can, in turn, impact propensity to drink and lay the groundwork for future addictive behavior, regulated by the striatum (Davis, 2008). BDNF is enriched in the striatum, delivered predominately via corticostriatal afferents, and BDNF polymorphisms have been linked to decreased synaptic plasticity involved in learning both complex and simple learning motor tasks (Cárdenas-Morales et al., 2014). It is possible that BDNF alterations in the striatum could impact synaptic plasticity or neurogenesis and could cause our observed complex motor learning changes.
Our experiment showed that chronic low/moderate gestational ethanol exposure had no effect on open field behavior or Barnes maze performance in outbred adult mice. Our results are in line with some previous studies that have not detected deficits that persist into adulthood for open field, learning/memory tasks, nor locomotor activity in inbred mouse models after chronic gestational ethanol exposure (Boehm et al., 2008; Downing et al., 2009), but it is also possible that our outbred strain introduces enough genetic variability to mask learning or open field phenotypes that have been previously found in inbred strains.
However, these ethanol-exposed offspring previously exhibited significantly altered timing to achieve surface righting, cliff aversion, and open field traversal developmental milestones as neonates (Chi et al., 2016), so our results may also indicate some sort of recovery or compensatory response by the central nervous system to the effects of early ethanol exposure has occurred. In most rodent FASD models, motor deficits are visible early in life and have disappeared by adulthood (Patten et al., 2014).
The value of non-inbred FASD models is currently being explored in other ways: High Alcohol Preferring mice and Low Alcohol Preferring mice have been developed by interbreeding eight inbred strains that display these drinking tendencies (Bice et al., 2011). Testing has shown differences in open field behavior between High and Low Alcohol Preferring mice in the absence of alcohol exposure (Can et al., 2012). Further behavioral characterization of these animals using an FASD model would provide useful motor behavior information, while decreasing the genetic variance present.
Future ethanol research should pay particular attention to adult phenotypes in order to document the persistence of these changes in FASD models, and to investigate compensatory mechanisms that may allow ethanol-exposed juveniles to overcome these deficits by adulthood. Neuroanatomical analysis should be performed at both juvenile and adult time points to detect possible apoptosis in brain areas involved in motor function. Apart from cell death, both cerebellar and striatal mechanisms that govern the execution of complex motor tasks should be examined, as well as possible long-lasting changes in synaptic plasticity or neurogenesis that may occur following developmental ethanol exposure.
F1000Research: Dataset 1. Raw data of motor learning deficit in adult FASD mice, 10.5256/f1000research.9237.d130218 (Reekes et al., 2016).
EC and all other authors conceived the study, designed the experiments, prepared all manuscript drafts, and have agreed to the final content. SB, MB, WE, TR, BB, and HW carried out the open field and Barnes maze experiments. TV, AE, ZT, and WM carried out the motor testing. All authors were involved in the revision of the draft manuscript. CF performed the gestational ethanol exposure and acted as a consultant.
A portion of this work was funded by a San Diego Instruments Rotarod Equipment Research Loan Award from the Faculty for Undergraduate Neuroscience to EC.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank John Reekes for logistical assistance and Jennie Jenkins for laboratory support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
References
1. Lazic SE, Essioux L: Improving basic and translational science by accounting for litter-to-litter variation in animal models.BMC Neurosci. 2013; 14: 37 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 01 Aug 16 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)