Introduction
Normal gastrointestinal (GI) function requires a system capable of adjusting to, at times, rapidly or dramatically shifting volumes due to food intake, fragmentation of larger ingested particles, and mixing and movement of chyme to bring nutrients to the absorptive sites and ultimately to expel residual materials from the gut. Many of these tasks depend on forces generated by the smooth muscle cells found in the mammalian gut. Abnormalities of GI motility, whether inherited, acquired, or induced by medications, may thus have significant implications on nutrient intake, transport, absorption, and fecal output. For this review, we will focus on one aspect of motor dysfunction: hypomotility. We will try to define underlying mechanisms, the consequences on the mammalian gut, and our ability to diagnose and treat it as a potential cause for disease. We will primarily use decreased contractile force as our operational definition of hypomotility, relate it to transit whenever possible, and focus on clinical aspects rather than the molecular or physiological mechanisms.
The mammalian, and thus also the human, gut has a basic structural organization that includes distinct muscle layers. The innermost muscularis mucosae separates the mucosa from the submucosa and likely contributes to the movement of chyme in the microenvironment close to the absorptive surfaces1. However, relatively little is known about its role in human disease. The more prominent and better-studied muscularis externa layer contains fibers oriented in circular and longitudinal directions that form its inner and outer component, respectively. For the purpose of this review, we will discuss hypomotility largely based on the assumption that contractile forces and patterns generated by this external layer play a key role in the tasks outlined above2.
Mechanisms of hypomotility
Considering the role of muscle activity, disorders of smooth muscle function, such as inherited abnormalities of contractile proteins, by definition contribute to the development of hypomotility3,4. Recent studies suggest that other molecular defects may lead to subtle, but potentially more common, manifestations. For example, detailed molecular and physiologic investigations identified changes in a voltage-sensitive sodium channel in patients with irritable bowel syndrome (IBS)5,6. In addition to inherited abnormalities or susceptibility, patients may also acquire changes involving contractile proteins, ion channels, or other molecules, as has been shown for diabetic gastroparesis7,8.
Muscle cells ultimately generate forces and create the motor events we can observe, but they are regulated by several cell types, which may be responsible for disorders characterized by hypomotility. The interstitial cells of Cajal form a network of functionally coupled cells within the muscle layer of the GI tract, where they generate and transmit electrical activity that controls smooth muscle function9. Animals with congenital absence of these cells do not display normal electrical activity and have significant abnormalities of motility and transit10. Consistent with experimental data, inherited and acquired, potentially reversible changes have been identified in various motility disorders10–12. Recent studies raise questions about a potential role of macrophages as modulators of GI motility. These macrophages form a three-dimensional network within the muscle layers and produce a variety of mediators that can alter gut function13,14.
Innervation plays an important role in the regulation of GI motility. The intrinsic or enteric nervous system forms the myenteric plexus with ganglia being located between the circular and longitudinal portions of the muscular layer. Localized abnormalities, such as inherited aganglionosis of the rectum (Hirschsprung’s disease) or acquired loss of ganglion cells in achalasia, disrupt the normal pattern of activity and, in the context of these disorders of sphincteric structures, delay or even block the passage of luminal contents15,16. Changes in enteric neurons or their function may also contribute to a variety of disorders characterized by abnormal motility, such as esophageal dysfunction in Sjögren’s syndrome, gastroparesis, pseudo-obstruction, or chronic constipation11,17–22. Extrinsic innervation provides a link between the central nervous system and the GI tract, often referred to as the brain-gut axis. Based on anatomic and functional criteria, extrinsic innervation is typically divided into sympathetic and parasympathetic components, with the vagus nerve being the predominant component of the parasympathetic branch of the autonomic nervous system. Experimental approaches have defined its important modulatory influences in many different areas, which range from motility and secretion to immune and endocrine function. The clinical impact can be seen in patients who have undergone a surgical vagotomy as a treatment of ulcer disease, which often leads to gastric atony, impaired opening of the pyloric channel, and prolonged retention of ingested material23. Vagotomies are rarely performed nowadays, but unintentional vagal injury during foregut surgery or autonomic neuropathy may contribute to the development of motility disorders, such as dyspeptic symptoms after anti-reflux surgery or diabetic gastroparesis24–26.
Inherited or acquired connective tissue disorders may manifest with impaired GI motility. The network of connective tissue provides the scaffolding for muscle cells. Few mechanistic studies systematically examined the exact physiological role of this passive support, yet the consequences of systemic sclerosis or inherited disorders, such as Marfan or Ehlers Danlos syndrome, clearly highlight its importance27–29. Other problems, such as vascular or joint manifestations, often predominate the classic manifestations of these rare diseases. However, less severe phenotypes, such as joint hypermobility syndrome, are more common and – as shown recently – seem to also be associated with a high prevalence of functional GI disorders30,31.
By far the most important, but not always fully appreciated, cause for changes in GI motility is the use of medications. A host of different agents can decrease contractile forces, change patterns of contractions, and/or delay transit of material throughout the gut. The list includes agents that are even available over the counter, such as anti-histamines or loperamide, but also prescription medications, such as opioids, calcium channel blockers, nitrates, anticholinergic substances, several antidepressants, antipsychotics, or anti-emetics, to mention just a few of the more common culprits.
Consequences of hypomotility
Considering the important role of normal GI motility in assuring entry into and transit through the gut with mixing and fragmentation also facilitating absorption, hypomotility should compromise normal GI function and nutritional status and/or cause symptoms. Consistent with these theoretical considerations and the previously mentioned role of medications as a cause of motor dysfunction, blunting contractile amplitudes through antimuscarinics, opioids, and L-type calcium channel blockers delays orocecal transit and alters meal-induced changes in colonic activity, likely contributing to the development of constipation that is often seen with these agents32–36. Conversely, cholinergic agonists increase contractile forces and improve esophageal clearance of swallowed fluids37,38. Independent of such pharmacologic investigations as proof of concept, detailed studies of esophageal motility clearly show the relevance of normal contractile function. A complete lack of normal peristaltic activity as an extreme case of hypomotility is very rare in asymptomatic individuals and is typically associated with dysphagia39. More limited changes with localized decrease in contractile amplitudes below 30 mmHg correlate with impaired bolus clearance if they involve a sufficiently long segment of the esophagus40,41 but may not necessarily trigger significant symptoms42–45.
This correlation between low contractile amplitudes as an operational definition of hypomotility and impaired transit or symptoms is even less consistent when we look at gastric function. While antimuscarinic agents slow gastric emptying in healthy volunteers46, the L-type calcium channel blockers nifedipine and verapamil have no effect47,48. Similarly, manipulation of transit with erythromycin or morphine in healthy persons does not correlate with consistent and significant changes in contractile indices measured with a wireless capsule49. Conversely, activation of cholinergic pathways with bethanechol or neostigmine increases contractile activity but does not accelerate gastric emptying46,50. Perhaps most importantly, neither emptying nor an aggregate measure of contractile activity, the motility index (MI), correlates consistently with symptoms, which may in part be due to limited coordination of contractions within functional units of the GI tract51–56. Increased activity triggered by a meal or pharmacologic stimulation is associated with higher ratings of dyspeptic symptoms50,51,57, pointing at the role of more complex motor patterns rather than measures of force generation only. Detailed physiological testing supports such a conclusion, as abnormal patterns are common in patients with severe GI dysfunction or functional dyspepsia58–60 and correlate with altered transit of ingested material through the GI tract61,62. While we have a more complex understanding of the many different factors controlling GI motility, the clinical manifestations and results of diagnostic testing still largely fit into the dichotomous concept of the previously proposed neurogenic or myogenic mechanism of dysmotility that separates abnormalities in patterns from those defined by abnormal amplitudes63.
Assessment of hypomotility
The very principles of functional GI testing were introduced more than 100 years ago, when gastric intubation with rubber tubes allowed the measurement of residual contents after a test meal, the first recordings of pressure changes, and – with the advent of radiography – the indirect visualization of contractions and emptying64. While the techniques have been refined and advanced significantly, they still focus on direct or indirect recording of contractions and the resulting movements of luminal contents (Figure 1). The direct assessment of contractile forces typically employs catheter-based systems and is thus invasive. Esophageal and anorectal manometry have become routine diagnostic tools in modern medicine; antroduodenal and colonic manometry require more complex instrumentation and prolonged recordings and have unclear diagnostic utility, which have limited their application in clinical practice. The miniaturization and wireless signal transfer brought us a capsule-based system that measures pH and pressure as the capsule is propelled through the gut. The pH allows us to approximate its location (the stomach is highly acidic, and fermentation lowers the pH to about 5 in the proximal colon). This technique has opened up options to non-invasively assess motor activity and entry/exit rather than transit per se65,66. Yet it provides limited information about motility patterns, which would require prolonged recordings from multiple sites in stable and predefined locations.
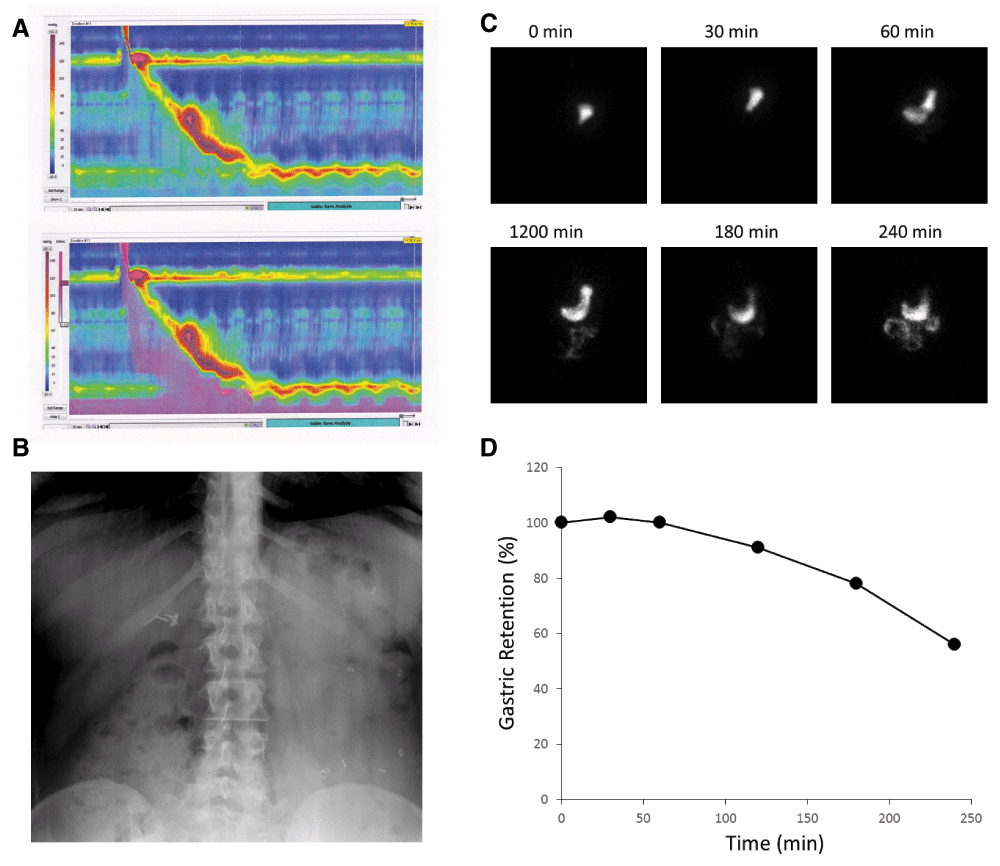
Figure 1. Examples of clinically used assessments of gastrointestinal (GI) motility.
Panel A demonstrates a pseudocolor display of esophageal pressure changes in response to a swallow (upper panel). The associated changes in impedance, caused by the traversing fluid bolus, are superimposed in purple in the lower panel. In Panel B, radio-opaque markers can be seen in the stool-filled colon (mostly accumulated on the left side) and allow an estimate of whole gut transit time. Gastric emptying of a radioactively labeled meal is documented with intermittent scintigraphic imaging (Panel C) and plotted as a function of time (Panel D).
Focusing on the movement of luminal content rather than contractions offers an alternative endpoint for clinical and scientific investigations of GI motility. The most commonly employed approaches largely rely on radioactive molecules that label physiologically relevant substrates that can be followed with scintigraphic methods (Figure 1C&D)67,68. For slower phenomena, such as the determination of whole gut transit, intermittent X-rays suffice to determine the number and location of retained radio-opaque markers (Figure 1B), which allows us to calculate an approximate transit time69,70. While not used as often, we can exploit changes in the microenvironment of different compartments within the GI tract to determine transit times. Early studies relied on the urinary excretion of mostly colored labels that were absorbed after reaching the small bowel. As this approach has urine production and bladder emptying as confounders, the temporal resolution is quite limited. Nowadays, the same principle typically uses substrates that are absorbed or fermented, diffuse across membranes, and ultimately reach the lungs where they are exhaled and can be easily captured in ‘real time’ to assess gastric emptying or orocecal transit71–73.
Conceptually, contractile activity and patterns will ultimately propel ingested material along the axis of the GI tract. Thus, both endpoints should relate to each other as shown for achalasia as an example in Figure 2. However, direct assessments suggest an, at times, poor correlation between overall test results of manometry and transit studies74. This led to the development of approaches that assess both parameters in parallel. The combination of pressure measurements and impedance changes after the ingestion of typically a liquid, but at times viscous bolus, has now become a routine test in clinical practice (Figure 1A)43,75. Simple constraints due to more difficult access, more complex motor pattern, and the need for longer recording times require a different strategy for the assessment of gastric, small bowel, or colonic contractions and transit measurements. While these strategies are not yet ready for routine application, investigators combined assessments of luminal filling and wall motions of segments within the GI tract using computerized reconstructions of cross-sectional imaging, such as magnetic resonance imaging (MRI). In the colon, dynamic MRI shows a good correspondence between high amplitude propagating pressure waves assessed manometrically and luminal diameter changes with fluid propulsion, triggered by intracolonic infusion of the laxative bisacodyl76. While many reports still refer to amplitudes of observed contractions, this analysis is based on wall motion or diameter changes with amplitudes being defined by geometric rather than pressure differences77–79. Parallel assessment at several points over time may enable the investigator to recognize patterns that can then be correlated with movement of luminal contents. Adding specifically designed magnetic markers may even allow us to directly measure transit or velocity of movement within the gut lumen80. While this is labor intensive and costly, initial results show promise in differentiating disease from health, separating between different disorders79,81, and identifying the effect of pharmacological interventions on contractile activity and movement of luminal content78,82. Thus, we have a proof of concept demonstrating that assessment of complex structure-function relationships is possible and may provide more insight into disease mechanisms or treatment options.
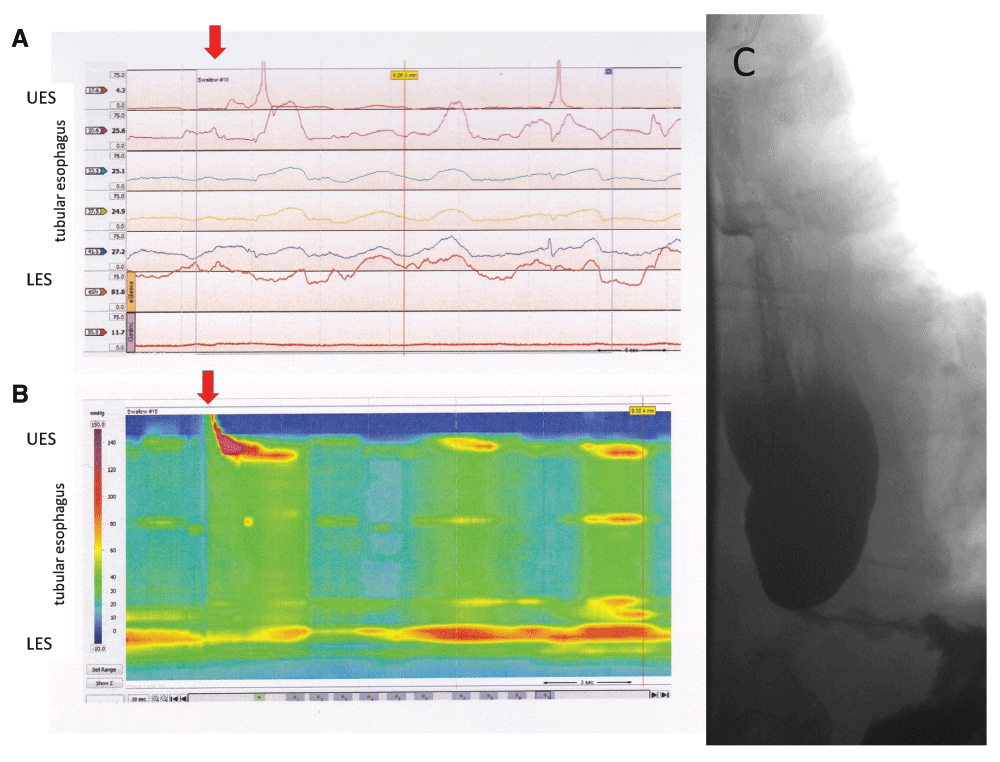
Figure 2. Different test results and their representation obtained in a patient with the esophageal motility disorder achalasia.
Pressure recordings obtained at different levels can be displayed as line tracings, showing the typical manometric results in this disorder with aperistalsis in the tubular esophagus and incomplete relaxations of the lower esophageal sphincter (LES) (Panel A). The same findings are shown as high-resolution esophageal pressure topography, with the results of many different recording sites being color-coded and with a seamless display of the entire esophageal length based on real and extrapolated data (Panel B). The corresponding contrast study (Panel C) shows a dilated esophagus with contrast retention and smooth tapering of the distal esophagus with a non-opening LES.
Treatment of hypomotility
During the last two decades, physicians and scientists have tried to translate our better understanding of GI motor function into better treatment for GI motor dysfunction. While we did indeed make progress, a retrospective viewpoint gives the process the appearance of a rollercoaster ride. The discovery of erythromycin’s effects as a potent motilin agonist gave physicians a prokinetic with effects on gastric and small bowel motility83,84. However, the unwanted antibiotic effects, the potential for adverse effects, and drug-drug interactions as well as an apparent loss of efficacy due to tachyphylaxis led to the development of several alternatives. Before looking at some of these alternatives, clinicians experimented with another macrolide, azithromycin, which had similar effects when studied acutely but comes with fewer drug-drug interactions than erythromycin. Despite this conceptual advantage, evaluations do not allow conclusions, as they were limited to short-term assessments with manometry85,86. More importantly, there are still the antibiotic properties as a major drawback. With the encouraging initial data, motilin agonists have surfaced and resurfaced since, with ABT-22987, KC 1145888, and mitemcinal all improving symptoms but not demonstrating true benefits when compared to placebo89,90. After a brief hiatus, another agent, camicinal, has been shown to accelerate gastric emptying in healthy volunteers without affecting esophageal, small bowel, or colonic transit91. While the jury is still out on the utility of this most recent agent, new observations may rekindle interest in motilin agonists. We typically use these agents to stimulate gastric motility and emptying, yet studies in healthy volunteers point at another potential use. When asked to rate symptoms during recordings of normal gastric motor activity, participants reported higher hunger scores during phase III of the cyclical activity pattern, seen in the fasting state92. This period of clustered and propagating activity fronts is associated with an increase in motilin, prompting follow-up experiments with motilin agonists. Interestingly, these agonists triggered clustered contractile fronts that propagated distally and heightened hunger feelings, which was not seen after the administration of a cholinergic agonist that simply increased the frequency and amplitude of contractions without a phase III-like activity. Conversely, the investigators also noted that unexplained anorexia is associated with loss of phase III93. With nutritional problems, most importantly the obesity epidemic we are facing, such observations may translate into novel applications in the future.
An extensive body of research has established the role of serotonin (5-HT) with its many different receptor subtypes in regulating gut function, motivating clinicians and drug companies to explore the therapeutic potential of serotoninergic agents. The initial results were promising. Cisapride, with its mixed agonistic and antagonistic properties, enhanced contractile forces and accelerated transit, even though symptomatic benefit was less consistent94–96. The agent was eventually withdrawn from the market, as interactions with an inwardly rectifying potassium channel prolonged the cardiac repolarization phase and led to torsade de pointes97. The convincing basic science and the observed effects of cisapride prompted the development of several other agents with more specific binding to the 5-HT4 receptor. Activation of this pathway facilitates acetylcholine release, which modifies intrinsic signaling and could potentially improve motor function and maintain physiologically relevant patterns98. While conceptually appealing and backed by strong preclinical studies, the track record of these agents is littered with problems. Alosetron, which did not target hypomotility but slowed down transit and was approved as a selective 5-HT3 antagonist for the management of diarrhea-predominant IBS, was withdrawn owing to an increase in cases of ischemic colitis99,100. Tegaserod, the first and only selective 5-HT4 agonist approved in the US, accelerated transit101 and improved constipation in women with or without IBS symptoms but was linked to an unexpected rise in myocardial infarction, leading to its withdrawal from the market102. Interestingly, while studies and marketing emphasized the benefit on pain and discomfort in IBS103,104, the only comparative effectiveness analysis studies did not show superiority over a simple osmotic laxative with a better risk profile105. Within the last few years, several newer agents have been tested in preclinical and clinical studies106–114. Concerns about side effects115,116, limited efficacy117–122, loss of efficacy over time123, and equivalence or even inferiority compared to cheaper and safer agents124–127 have continued to raise questions about the cost-benefit ratio and true utility of this class of agents. Given that abnormalities in 5-HT levels and release are linked to IBS, perhaps more direct targeting of 5-HT release is worthwhile.
Cholinergic agents have been available for a long time, with atropine and hyoscyamine being part of the 'pharmacopoeia' of physicians for centuries. Considering the role of acetylcholine in neuromuscular transmission, it makes intuitive sense to use agonists as a treatment for hypomotility. For acute colonic pseudo-obstruction, enhancing cholinergic signaling has indeed become the first-line therapy128. In the esophagus, contractile amplitudes increase37,38. However, despite these acute effects on esophageal physiology, there was no tangible benefit in terms of reflux symptoms or acid exposure129. Studies on dysphagia are largely restricted to the management of myasthenia gravis, which demonstrate a benefit of cholinesterase inhibitors but obviously target neuromuscular transmission of skeletal muscle affected in this disorder. Similarly, gastric contractions increased after administration of cholinergic agonists46,130, yet emptying did not improve and symptoms may even worsen46. In diabetic patients, the cholinesterase inhibitor pyridostigmine similarly did not alter gastric emptying but showed a benefit in patients with chronic constipation131,132. Thus, the picture is at best mixed and highlights that patterns rather than simple contractile forces generated play an important role in normal gut function.
In the last decade, observations related to the peptide hormone ghrelin have generated quite a bit of interest about its possible utility in patients with impaired gastric function or dyspepsia. Levels rise prior to food intake, regulate appetite, and modulate gastric emptying133,134. Initial experiments were encouraging, as they showed that infusion of ghrelin or short-term use of ghrelin agonists improved dyspeptic symptoms and gastric emptying135–137. Effects seemed to go beyond the regulation of gastric motility, as the ghrelin agonist relamorelin increased meal-induced propagating contractions and accelerated colonic transit in small trials of patients with constipation138,139. Another ghrelin agonist, ulimorelin (TZP 101), shortened the time to first bowel movement after partial colectomy in an initial dose-finding study140 but was not superior to placebo in the larger trials on postoperative ileus141. Initial studies of this agent in gastroparesis were similarly promising137,142,143. However, further development of its oral analogue has been halted after larger trials did not show benefit over placebo in gastroparesis144,145. Dyspeptic symptoms, such as nausea, and impaired gastric emptying have been linked to changes in electrical activity that can be recorded from the human stomach60,146,147. These observations led to the idea that implantation of stimulation electrodes may entrain the basic electrical rhythm, thereby indirectly improving coordination of contractile activity, emptying, and ultimately dyspeptic symptoms. Initial experiments with temporarily implanted electrodes demonstrated the feasibility in humans and motivated the development of systems that could be permanently implanted for gastric electrical stimulation (‘gastric pacers’)148–150. Subsequent experiments optimized the stimulation parameters, which now employ a high frequency that does not target gastric muscles and motility, is not associated with consistent changes in emptying, and presumably works through modulation of afferent input151. While open label studies suggested significant benefit, controlled trials did not support superiority over sham stimulation152.
Questions and directions for future research
Using increasingly sophisticated techniques, we can now assess contractile forces, patterns, and transit in the various functionally distinct compartments of the GI tract. However, we are still lacking reliable diagnostic and predictive markers. In patients with dyspeptic symptoms, delays in gastric emptying and their treatment-associated changes do not correlate with symptom improvement153. Considering the complex responses to food intake, we may need to shift from a focus on facilitating emptying and consider other mechanisms, such as impaired accommodation154. A more nuanced assessment of symptoms or mechanisms may enable us to target a subset of symptoms, as, for example, for postprandial bloating, which tends to respond better to prokinetics155,156. While antroduodenal or colonic manometry may predict treatment responses in subsets of patients157,158, the true utility of these assessments has never been examined systematically, with conclusions being largely based on small cohort studies of skewed patient groups. As described in this article, we have focused on physiologic variables when approaching patients with symptoms and possible gut dysfunction. However, recent studies of functional illnesses defined by altered motility or transit show the impact of psychological factors as confounders, which may not only influence the perceived symptom severity but also drive healthcare-seeking behavior159–163 or predict treatment outcomes164. Considering the conceptual importance of brain-gut interactions, future studies need to define to what extent these correlations are consequences of altered physiology or whether the GI manifestations are primarily somatic manifestations of emotional or other psychiatric problems.
Several decades after the introduction of functional testing into clinical practice, we still have limited options to assess small bowel and colonic function. The wireless motility capsule may well point in the right direction, but it still provides too little insight, considering the importance of motility patterns discussed above. Nanoparticles, perhaps combined with specifically designed adhesive gels, may allow non-invasive monitoring of motor activity, patterns, and relevant changes in the local microenvironment165. Such approaches may prove useful beyond diagnostic strategies, as they could be combined with focused/localized drug delivery.
The last few years have clearly moved our attention to a previously often-neglected component of the luminal contents: microbial colonization. Several interesting studies have emerged and should attract our attention. Perhaps not surprisingly, changes in nutrient-microbiome interactions altered GI transit in a mouse model, which is in part driven by poorly absorbed materials and thus confounded by fermentation and likely increases in the volume of colonic contents166,167. However, volume changes explain only a part of this complex interplay, as specific microbial species and their metabolites modulate serotonin content in enteroendocrine cells, which in turn affect gut function168,169. While probiotics have long been a part of medical management, their effects are still limited170, showing the need for a better understanding of the triad of food, gut, and microbes.
Electrical stimulation found its way into the medical management of GI disorders more than a century ago64. The results of gastric electrical stimulation described above show continued shortcomings. Despite ongoing problems, similar approaches were recently tried in constipation. Early open label studies reported promising results171 but also raised concerns about long-term efficacy and safety with high rates of problems and reoperations, which have been shown in sacral neurostimulation for other indications172.
GI motility matters. It allows us to ingest and digest food and ultimately expel the residue. Normal motor function relies on the complex interplay of the central and peripheral nervous system, different cell types in the GI muscle wall, and the luminal contents. Decreasing forces or altering patterns of normal contractions, the correlates of ‘hypomotility’, can interfere with transit, lead to symptoms, and/or compromise nutritional status. New development may enable us to better measure and analyze contractile forces, patterns, and transit, use them more effectively as biomarkers of hypomotility, identify new targets for our interventions, and understand the complex relationship that emerges from the brain-gut axis and closely links emotion with gut function and symptoms that ultimately determine quality of life.
Competing interests
The authors declare that they have no competing interests.
Grant information
The author(s) declared that no grants were involved in supporting this work.
Faculty Opinions recommendedReferences
- 1.
Uchida K, Kamikawa Y:
Muscularis mucosae - the forgotten sibling.
J Smooth Muscle Res.
2007; 43(5): 157–77. PubMed Abstract
| Publisher Full Text
- 2.
Sanders KM, Koh SD, Ro S, et al.:
Regulation of gastrointestinal motility--insights from smooth muscle biology.
Nat Rev Gastroenterol Hepatol.
2012; 9(11): 633–45. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 3.
Lehtonen HJ, Sipponen T, Tojkander S, et al.:
Segregation of a missense variant in enteric smooth muscle actin γ-2 with autosomal dominant familial visceral myopathy.
Gastroenterology.
2012; 143(6): 1482–1491.e3. PubMed Abstract
| Publisher Full Text
- 4.
Klar J, Raykova D, Gustafson E, et al.:
Phenotypic expansion of visceral myopathy associated with ACTG2 tandem base substitution.
Eur J Hum Genet.
2015; 23(12): 1679–83. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 5.
Verstraelen TE, Ter Bekke RM, Volders PG, et al.:
The role of the SCN5A-encoded channelopathy in irritable bowel syndrome and other gastrointestinal disorders.
Neurogastroenterol Motil.
2015; 27(7): 906–13. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 6.
Beyder A, Mazzone A, Strege PR, et al.:
Loss-of-function of the voltage-gated sodium channel NaV1.5 (channelopathies) in patients with irritable bowel syndrome.
Gastroenterology.
2014; 146(7): 1659–68. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 7.
Bhetwal BP, An C, Baker SA, et al.:
Impaired contractile responses and altered expression and phosphorylation of Ca2+ sensitization proteins in gastric antrum smooth muscles from ob/ob mice.
J Muscle Res Cell Motil.
2013; 34(2): 137–49. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 8.
Li S, Chen JD:
Decreased L-type calcium current in antral smooth muscle cells of STZ-induced diabetic rats.
Neurogastroenterol Motil.
2014; 26(7): 971–9. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 9.
Sanders KM, Ward SM, Koh SD:
Interstitial cells: regulators of smooth muscle function.
Physiol Rev.
2014; 94(3): 859–907. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 10.
Ward SM, Sanders KM:
Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks.
Am J Physiol Gastrointest Liver Physiol.
2001; 281(3): G602–11. PubMed Abstract
- 11.
Grover M, Bernard CE, Pasricha PJ, et al.:
Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium.
Neurogastroenterol Motil.
2012; 24(6): 531–9, e249. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 12.
Mazzone A, Bernard CE, Strege PR, et al.:
Altered expression of Ano1 variants in human diabetic gastroparesis.
J Biol Chem.
2011; 286(15): 13393–403. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 13.
Bernard CE, Gibbons SJ, Mann IS, et al.:
Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis.
Neurogastroenterol Motil.
2014; 26(9): 1275–84. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 14.
Neshatian L, Gibbons SJ, Farrugia G:
Macrophages in diabetic gastroparesis--the missing link?
Neurogastroenterol Motil.
2015; 27(1): 7–18. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 15.
Taketomi T, Yoshiga D, Taniguchi K, et al.:
Loss of mammalian Sprouty2 leads to enteric neuronal hyperplasia and esophageal achalasia.
Nat Neurosci.
2005; 8(7): 855–7. PubMed Abstract
| Publisher Full Text
- 16.
Furness JB:
The enteric nervous system and neurogastroenterology.
Nat Rev Gastroenterol Hepatol.
2012; 9(5): 286–94. PubMed Abstract
| Publisher Full Text
- 17.
Sjogren RW:
Gastrointestinal motility disorders in scleroderma.
Arthritis Rheum.
1994; 37(9): 1265–82. PubMed Abstract
| Publisher Full Text
- 18.
Goldblatt F, Gordon TP, Waterman SA:
Antibody-mediated gastrointestinal dysmotility in scleroderma.
Gastroenterology.
2002; 123(4): 1144–50. PubMed Abstract
| Publisher Full Text
- 19.
Kovács L, Marczinovits I, György A, et al.:
Clinical associations of autoantibodies to human muscarinic acetylcholine receptor 3213-228 in primary Sjogren's syndrome.
Rheumatology (Oxford).
2005; 44(8): 1021–5. PubMed Abstract
| Publisher Full Text
- 20.
Grover M, Farrugia G, Lurken MS, et al.:
Cellular changes in diabetic and idiopathic gastroparesis.
Gastroenterology.
2011; 140(5): 1575–85.e8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 21.
Verne GN, Sallustio JE, Eaker EY:
Anti-myenteric neuronal antibodies in patients with achalasia. A prospective study.
Dig Dis Sci.
1997; 42(2): 307–13. PubMed Abstract
| Publisher Full Text
- 22.
De Giorgio R, Guerrini S, Barbara G, et al.:
Inflammatory neuropathies of the enteric nervous system.
Gastroenterology.
2004; 126(7): 1872–83. PubMed Abstract
| Publisher Full Text
- 23.
Cowley DJ, Vernon P, Jones T, et al.:
Gastric emptying of solid meals after truncal vagotomy and pyloroplasty in human subjects.
Gut.
1972; 13(3): 176–81. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 24.
Ouyang H, Yin J, Wang Z, et al.:
Electroacupuncture accelerates gastric emptying in association with changes in vagal activity.
Am J Physiol Gastrointest Liver Physiol.
2002; 282(2):G390–6. PubMed Abstract
| Publisher Full Text
- 25.
Lee PG, Cai F, Helke CJ:
Streptozotocin-induced diabetes reduces retrograde axonal transport in the afferent and efferent vagus nerve.
Brain Res.
2002; 941(1–2): 127–36. PubMed Abstract
| Publisher Full Text
- 26.
Ardila-Hani A, Arabyan M, Waxman A, et al.:
Severity of dyspeptic symptoms correlates with delayed and early variables of gastric emptying.
Dig Dis Sci.
2013; 58(2): 478–87. PubMed Abstract
| Publisher Full Text
- 27.
Domsic R, Fasanella K, Bielefeldt K:
Gastrointestinal manifestations of systemic sclerosis.
Dig Dis Sci.
2008; 53(5): 1163–74. PubMed Abstract
| Publisher Full Text
- 28.
Nelson AD, Mouchli MA, Valentin N, et al.:
Ehlers Danlos syndrome and gastrointestinal manifestations: a 20-year experience at Mayo Clinic.
Neurogastroenterol Motil.
2015; 27(11): 1657–66. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 29.
Eliashar R, Sichel JY, Biron A, et al.:
Multiple gastrointestinal complications in Marfan syndrome.
Postgrad Med J.
1998; 74(874): 495–7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 30.
Fikree A, Aktar R, Grahame R, et al.:
Functional gastrointestinal disorders are associated with the joint hypermobility syndrome in secondary care: a case-control study.
Neurogastroenterol Motil.
2015; 27(4): 569–79. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 31.
Kovacic K, Chelimsky TC, Sood MR, et al.:
Joint hypermobility: a common association with complex functional gastrointestinal disorders.
J Pediatr.
2014; 165(5): 973–8. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 32.
Bassotti G, Calcara C, Annese V, et al.:
Nifedipine and verapamil inhibit the sigmoid colon myoelectric response to eating in healthy volunteers.
Dis Colon Rectum.
1998; 41(3): 377–80. PubMed Abstract
| Publisher Full Text
- 33.
Chiarioni G, Scattolini C, Bonfante F, et al.:
Effect of nifedipine on mouth-to-cecum transit of liquid meal in normal subjects.
Dig Dis Sci.
1993; 38(6): 1022–5. PubMed Abstract
| Publisher Full Text
- 34.
Meek PD, Evang SD, Tadrous M, et al.:
Overactive bladder drugs and constipation: a meta-analysis of randomized, placebo-controlled trials.
Dig Dis Sci.
2011; 56(1): 7–18. PubMed Abstract
| Publisher Full Text
- 35.
Wade PR, Palmer JM, McKenney S, et al.:
Modulation of gastrointestinal function by MuDelta, a mixed μ opioid receptor agonist/ μ opioid receptor antagonist.
Br J Pharmacol.
2012; 167(5): 1111–25. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 36.
Lecchini S, Marcoli M, De Ponti F, et al.:
Selectivity of Ca2+ channel blockers in inhibiting muscular and nerve activities in isolated colon.
Br J Pharmacol.
1991; 102(3): 735–41. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 37.
Blonski W, Vela MF, Freeman J, et al.:
The effect of oral buspirone, pyridostigmine, and bethanechol on esophageal function evaluated with combined multichannel esophageal impedance-manometry in healthy volunteers.
J Clin Gastroenterol.
2009; 43(3): 253–60. PubMed Abstract
| Publisher Full Text
- 38.
Agrawal A, Hila A, Tutuian R, et al.:
Bethanechol improves smooth muscle function in patients with severe ineffective esophageal motility.
J Clin Gastroenterol.
2007; 41(4): 366–70. PubMed Abstract
| Publisher Full Text
- 39.
Burgess NG, Wyeth JW:
An audit of combined multichannel intraluminal impedance manometry in the assessment of dysphagia.
J Gastroenterol Hepatol.
2011; 26(Suppl 3): 79–82. PubMed Abstract
| Publisher Full Text
- 40.
Lin Z, Nicodème F, Lin CY, et al.:
Parameters for quantifying bolus retention with high-resolution impedance manometry.
Neurogastroenterol Motil.
2014; 26(7): 929–36. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 41.
Omari TI, Szczesniak MM, Maclean J, et al.:
Correlation of esophageal pressure-flow analysis findings with bolus transit patterns on videofluoroscopy.
Dis Esophagus.
2016; 29(2): 166–73. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 42.
Bulsiewicz WJ, Kahrilas PJ, Kwiatek MA, et al.:
Esophageal pressure topography criteria indicative of incomplete bolus clearance: a study using high-resolution impedance manometry.
Am J Gastroenterol.
2009; 104(11): 2721–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 43.
Roman S, Lin Z, Kwiatek MA, et al.:
Weak peristalsis in esophageal pressure topography: classification and association with Dysphagia.
Am J Gastroenterol.
2011; 106(2): 349–56. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 44.
Ravi K, Friesen L, Issaka R, et al.:
Long-term Outcomes of Patients With Normal or Minor Motor Function Abnormalities Detected by High-resolution Esophageal Manometry.
Clin Gastroenterol Hepatol.
2015; 13(8): 1416–23. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 45.
Bogte A, Bredenoord AJ, Oors J, et al.:
Sensation of stasis is poorly correlated with impaired esophageal bolus transport.
Neurogastroenterol Motil.
2014; 26(4): 538–45. PubMed Abstract
| Publisher Full Text
- 46.
Parkman HP, Trate DM, Knight LC, et al.:
Cholinergic effects on human gastric motility.
Gut.
1999; 45(3): 346–54. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 47.
Yavorski RT, Hallgren SE, Blue PW:
Effects of verapamil and diltiazem on gastric emptying in normal subjects.
Dig Dis Sci.
1991; 36(9): 1274–6. PubMed Abstract
| Publisher Full Text
- 48.
Traube M, Lange RC, McAllister RG Jr, et al.:
Effect of nifedipine on gastric emptying in normal subjects.
Dig Dis Sci.
1985; 30(8): 710–2. PubMed Abstract
| Publisher Full Text
- 49.
Rozov-Ung I, Mreyoud A, Moore J, et al.:
Detection of drug effects on gastric emptying and contractility using a wireless motility capsule.
BMC Gastroenterol.
2014; 14: 2. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 50.
Di Stefano M, Vos R, Klersy C, et al.:
Neostigmine-induced postprandial phasic contractility in the proximal stomach and dyspepsia-like symptoms in healthy volunteers.
Am J Gastroenterol.
2006; 101(12): 2797–804. PubMed Abstract
| Publisher Full Text
- 51.
Simrén M, Vos R, Janssens J, et al.:
Unsuppressed postprandial phasic contractility in the proximal stomach in functional dyspepsia: relevance to symptoms.
Am J Gastroenterol.
2003; 98(10): 2169–75. PubMed Abstract
| Publisher Full Text
- 52.
Talley NJ, Verlinden M, Jones M:
Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility-like dyspepsia?
Am J Gastroenterol.
2001; 96(5): 1422–8. PubMed Abstract
| Publisher Full Text
- 53.
Di Stefano M, Miceli E, Tana P, et al.:
Fasting and postprandial gastric sensorimotor activity in functional dyspepsia: postprandial distress vs. epigastric pain syndrome.
Am J Gastroenterol.
2014; 109(10): 1631–9. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 54.
Barshop K, Staller K, Semler J, et al.:
Duodenal rather than antral motility contractile parameters correlate with symptom severity in gastroparesis patients.
Neurogastroenterol Motil.
2015; 27(3): 339–46. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 55.
Kloetzer L, Chey WD, McCallum RW, et al.:
Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule.
Neurogastroenterol Motil.
2010; 22(5): 527–33, e117. PubMed Abstract
| Publisher Full Text
- 56.
Angeli TR, Cheng LK, Du P, et al.:
Loss of Interstitial Cells of Cajal and Patterns of Gastric Dysrhythmia in Patients With Chronic Unexplained Nausea and Vomiting.
Gastroenterology.
2015; 149(1): 56–66.e5. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 57.
Noor N, Small PK, Loudon MA, et al.:
Effects of cisapride on symptoms and postcibal small-bowel motor function in patients with irritable bowel syndrome.
Scand J Gastroenterol.
1998; 33(6): 605–11. PubMed Abstract
| Publisher Full Text
- 58.
Cogliandro RF, Antonucci A, De Giorgio R, et al.:
Patient-reported outcomes and gut dysmotility in functional gastrointestinal disorders.
Neurogastroenterol Motil.
2011; 23(12): 1084–91. PubMed Abstract
| Publisher Full Text
- 59.
Wilmer A, Van Cutsem E, Andrioli A, et al.:
Ambulatory gastrojejunal manometry in severe motility-like dyspepsia: lack of correlation between dysmotility, symptoms, and gastric emptying.
Gut.
1998; 42(2): 235–42. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 60.
Sha W, Pasricha PJ, Chen JD:
Correlations among electrogastrogram, gastric dysmotility, and duodenal dysmotility in patients with functional dyspepsia.
J Clin Gastroenterol.
2009; 43(8): 716–22. PubMed Abstract
| Publisher Full Text
- 61.
Dinning PG, Szczesniak MM, Cook IJ:
Proximal colonic propagating pressure waves sequences and their relationship with movements of content in the proximal human colon.
Neurogastroenterol Motil.
2008; 20(5): 512–20. PubMed Abstract
| Publisher Full Text
- 62.
Cook IJ, Furukawa Y, Panagopoulos V, et al.:
Relationships between spatial patterns of colonic pressure and individual movements of content.
Am J Physiol Gastrointest Liver Physiol.
2000; 278(2): G329–41. PubMed Abstract
- 63.
Greydanus MP, Camilleri M:
Abnormal postcibal antral and small bowel motility due to neuropathy or myopathy in systemic sclerosis.
Gastroenterology.
1989; 96(1): 110–5. PubMed Abstract
| Publisher Full Text
- 64.
Bielefeldt K:
From ischochymia to gastroparesis: proposed mechanisms and preferred management of dyspepsia over the centuries.
Dig Dis Sci.
2014; 59(6): 1088–98. PubMed Abstract
| Publisher Full Text
- 65.
Lee A, Wilding G, Kuo B:
Variable abnormal physiological motility in the proximal upper gastrointestinal tract in gastroparesis.
Neurogastroenterol Motil.
2012; 24(7): 652–7, e276. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 66.
Maqbool S, Parkman HP, Friedenberg FK:
Wireless capsule motility: comparison of the SmartPill GI monitoring system with scintigraphy for measuring whole gut transit.
Dig Dis Sci.
2009; 54(10): 2167–74. PubMed Abstract
| Publisher Full Text
- 67.
Guo JP, Maurer AH, Fisher RS, et al.:
Extending gastric emptying scintigraphy from two to four hours detects more patients with gastroparesis.
Dig Dis Sci.
2001; 46(1): 24–9. PubMed Abstract
| Publisher Full Text
- 68.
Pathikonda M, Sachdeva P, Malhotra N, et al.:
Gastric emptying scintigraphy: is four hours necessary?
J Clin Gastroenterol.
2012; 46(3): 209–15. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 69.
Olausson EA, Brock C, Drewes AM, et al.:
Measurement of gastric emptying by radiopaque markers in patients with diabetes: correlation with scintigraphy and upper gastrointestinal symptoms.
Neurogastroenterol Motil.
2013; 25(3): e224–32. PubMed Abstract
| Publisher Full Text
- 70.
Rao SS, Coss-Adame E, Valestin J, et al.:
Evaluation of constipation in older adults: radioopaque markers (ROMs) versus wireless motility capsule (WMC).
Arch Gerontol Geriatr.
2012; 55(2): 289–94. PubMed Abstract
| Publisher Full Text
- 71.
Choi MG, Camilleri M, Burton DD, et al.:
Reproducibility and simplification of 13C-octanoic acid breath test for gastric emptying of solids.
Am J Gastroenterol.
1998; 93(1): 92–8. PubMed Abstract
| Publisher Full Text
- 72.
Delbende B, Perri F, Couturier O, et al.:
13C-octanoic acid breath test for gastric emptying measurement.
Eur J Gastroenterol Hepatol.
2000; 12(1): 85–91. PubMed Abstract
| Publisher Full Text
- 73.
Zhao J, Zheng X, Chu H, et al.:
A study of the methodological and clinical validity of the combined lactulose hydrogen breath test with scintigraphic oro-cecal transit test for diagnosing small intestinal bacterial overgrowth in IBS patients.
Neurogastroenterol Motil.
2014; 26(6): 794–802. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 74.
Mugie SM, Perez ME, Burgers R, et al.:
Colonic manometry and colonic scintigraphy as a diagnostic tool for children with severe constipation.
J Pediatr Gastroenterol Nutr.
2013; 57(5): 598–602. PubMed Abstract
| Publisher Full Text
- 75.
Xiao Y, Kahrilas PJ, Kwasny MJ, et al.:
High-resolution manometry correlates of ineffective esophageal motility.
Am J Gastroenterol.
2012; 107(11): 1647–54. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 76.
Kirchhoff S, Nicolaus M, Schirra J, et al.:
Assessment of colon motility using simultaneous manometric and functional cine-MRI analysis: preliminary results.
Abdom Imaging.
2011; 36(1): 24–30. PubMed Abstract
| Publisher Full Text
- 77.
Baba S, Sasaki A, Nakajima J, et al.:
Assessment of gastric motor function by cine magnetic resonance imaging.
J Gastroenterol Hepatol.
2009; 24(8): 1401–6. PubMed Abstract
| Publisher Full Text
- 78.
Menys A, Taylor SA, Emmanuel A, et al.:
Global small bowel motility: assessment with dynamic MR imaging.
Radiology.
2013; 269(2): 443–50. PubMed Abstract
| Publisher Full Text
- 79.
Ohkubo H, Kessoku T, Fuyuki A, et al.:
Assessment of small bowel motility in patients with chronic intestinal pseudo-obstruction using cine-MRI.
Am J Gastroenterol.
2013; 108(7): 1130–9. PubMed Abstract
| Publisher Full Text
- 80.
Chaddock G, Lam C, Hoad CL, et al.:
Novel MRI tests of orocecal transit time and whole gut transit time: studies in normal subjects.
Neurogastroenterol Motil.
2014; 26(2): 205–14. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 81.
Bharucha AE, Manduca A, Lake DS, et al.:
Gastric motor disturbances in patients with idiopathic rapid gastric emptying.
Neurogastroenterol Motil.
2011; 23(7): 617–e252. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 82.
Marciani L, Garsed KC, Hoad CL, et al.:
Stimulation of colonic motility by oral PEG electrolyte bowel preparation assessed by MRI: comparison of split vs single dose.
Neurogastroenterol Motil.
2014; 26(10): 1426–36. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 83.
Tomomasa T, Kuroume T, Arai H, et al.:
Erythromycin induces migrating motor complex in human gastrointestinal tract.
Dig Dis Sci.
1986; 31(2): 157–61. PubMed Abstract
| Publisher Full Text
- 84.
Janssens J, Peeters TL, Vantrappen G, et al.:
Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies.
N Engl J Med.
1990; 322(15): 1028–31. PubMed Abstract
| Publisher Full Text
- 85.
Moshiree B, McDonald R, Hou W, et al.:
Comparison of the effect of azithromycin versus erythromycin on antroduodenal pressure profiles of patients with chronic functional gastrointestinal pain and gastroparesis.
Dig Dis Sci.
2010; 55(3): 675–83. PubMed Abstract
| Publisher Full Text
- 86.
Chini P, Toskes PP, Waseem S, et al.:
Effect of azithromycin on small bowel motility in patients with gastrointestinal dysmotility.
Scand J Gastroenterol.
2012; 47(4): 422–7. PubMed Abstract
| Publisher Full Text
- 87.
Talley NJ, Verlinden M, Geenen DJ, et al.:
Effects of a motilin receptor agonist (ABT-229) on upper gastrointestinal symptoms in type 1 diabetes mellitus: a randomised, double blind, placebo controlled trial.
Gut.
2001; 49(3): 395–401. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 88.
Russo A, Stevens JE, Giles N, et al.:
Effect of the motilin agonist KC 11458 on gastric emptying in diabetic gastroparesis.
Aliment Pharmacol Ther.
2004; 20(3): 333–8. PubMed Abstract
| Publisher Full Text
- 89.
McCallum RW, Cynshi O, Investigative Team: Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis - a randomized, multicentre, placebo-controlled study.
Aliment Pharmacol Ther.
2007; 26(8): 1121–30. PubMed Abstract
| Publisher Full Text
- 90.
McCallum RW, Cynshi O, US Investigative Team: Efficacy of mitemcinal, a motilin agonist, on gastrointestinal symptoms in patients with symptoms suggesting diabetic gastropathy: a randomized, multi-center, placebo-controlled trial.
Aliment Pharmacol Ther.
2007; 26(1): 107–16. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 91.
Hobson R, Farmer AD, Dewit OE, et al.:
The effects of camicinal, a novel motilin agonist, on gastro-esophageal function in healthy humans-a randomized placebo controlled trial.
Neurogastroenterol Motil.
2015; 27(11): 1629–37. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 92.
Deloose E, Vos R, Corsetti M, et al.:
Endogenous motilin, but not ghrelin plasma levels fluctuate in accordance with gastric phase III activity of the migrating motor complex in man.
Neurogastroenterol Motil.
2015; 27(1): 63–71. PubMed Abstract
| Publisher Full Text
- 93.
Tack J, Deloose E, Ang D, et al.:
Motilin-induced gastric contractions signal hunger in man.
Gut.
2016; 65(2): 214–24. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 94.
Corinaldesi R, Stanghellini V, Raiti C, et al.:
Effect of chronic administration of cisapride on gastric emptying of a solid meal and on dyspeptic symptoms in patients with idiopathic gastroparesis.
Gut.
1987; 28(3): 300–5. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 95.
Richards RD, Valenzuela GA, Davenport KG, et al.:
Objective and subjective results of a randomized, double-blind, placebo-controlled trial using cisapride to treat gastroparesis.
Dig Dis Sci.
1993; 38(5): 811–6. PubMed Abstract
| Publisher Full Text
- 96.
Fraser RJ, Horowitz M, Maddox AF, et al.:
Postprandial antropyloroduodenal motility and gastric emptying in gastroparesis--effects of cisapride.
Gut.
1994; 35(2): 172–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 97.
Layton D, Key C, Shakir SA:
Prolongation of the QT interval and cardiac arrhythmias associated with cisapride: limitations of the pharmacoepidemiological studies conducted and proposals for the future.
Pharmacoepidemiol Drug Saf.
2003; 12(1): 31–40. PubMed Abstract
| Publisher Full Text
- 98.
Gallego D, Ortega O, Arenas C, et al.:
The effect of levosulpiride on in vitro motor patterns in the human gastric fundus, antrum, and jejunum.
Neurogastroenterol Motil.
2016; 28(6): 879–90. PubMed Abstract
| Publisher Full Text
- 99.
Lisi DM:
Lotronex withdrawal.
Arch Intern Med.
2002; 162(1): 101. PubMed Abstract
| Publisher Full Text
- 100.
Chang L, Tong K, Ameen V:
Ischemic colitis and complications of constipation associated with the use of alosetron under a risk management plan: clinical characteristics, outcomes, and incidences.
Am J Gastroenterol.
2010; 105(4): 866–75. PubMed Abstract
| Publisher Full Text
- 101.
Prather CM, Camilleri M, Zinsmeister AR, et al.:
Tegaserod accelerates orocecal transit in patients with constipation-predominant irritable bowel syndrome.
Gastroenterology.
2000; 118(3): 463–8. PubMed Abstract
| Publisher Full Text
- 102.
Pasricha PJ:
Desperately seeking serotonin… A commentary on the withdrawal of tegaserod and the state of drug development for functional and motility disorders.
Gastroenterology.
2007; 132(7): 2287–90. PubMed Abstract
| Publisher Full Text
- 103.
Müller-Lissner SA, Fumagalli I, Bardhan KD, et al.:
Tegaserod, a 5-HT4 receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation.
Aliment Pharmacol Ther.
2001; 15(10): 1655–66. PubMed Abstract
| Publisher Full Text
- 104.
Bardhan KD, Forbes A, Marsden CL, et al.:
The effects of withdrawing tegaserod treatment in comparison with continuous treatment in irritable bowel syndrome patients with abdominal pain/discomfort, bloating and constipation: a clinical study.
Aliment Pharmacol Ther.
2004; 20(2): 213–22. PubMed Abstract
| Publisher Full Text
- 105.
Di Palma JA, Cleveland MV, McGowan J, et al.:
A randomized, multicenter comparison of polyethylene glycol laxative and tegaserod in treatment of patients with chronic constipation.
Am J Gastroenterol.
2007; 102(9): 1964–71. PubMed Abstract
| Publisher Full Text
- 106.
Emmanuel AV, Roy AJ, Nicholls TJ, et al.:
Prucalopride, a systemic enterokinetic, for the treatment of constipation.
Aliment Pharmacol Ther.
2002; 16(7): 1347–56. PubMed Abstract
| Publisher Full Text
- 107.
De Maeyer JH, Lefebvre RA, Schuurkes JA:
5-HT4 receptor agonists: similar but not the same.
Neurogastroenterol Motil.
2008; 20(2): 99–112. PubMed Abstract
| Publisher Full Text
- 108.
Shimatani H, Kojima Y, Kadowaki M, et al.:
A 5-HT4 agonist mosapride enhances rectorectal and rectoanal reflexes in guinea pigs.
Am J Physiol Gastrointest Liver Physiol.
2003; 285(2): G389–95. PubMed Abstract
| Publisher Full Text
- 109.
Liu Z, Sakakibara R, Odaka T, et al.:
Mosapride citrate, a novel 5-HT4 agonist and partial 5-HT3 antagonist, ameliorates constipation in parkinsonian patients.
Mov Disord.
2005; 20(6): 680–6. PubMed Abstract
| Publisher Full Text
- 110.
Holtmann G, Talley NJ, Liebregts T, et al.:
A placebo-controlled trial of itopride in functional dyspepsia.
N Engl J Med.
2006; 354(8): 832–40. PubMed Abstract
| Publisher Full Text
- 111.
Goldberg M, Li YP, Johanson JF, et al.:
Clinical trial: the efficacy and tolerability of velusetrag, a selective 5-HT4 agonist with high intrinsic activity, in chronic idiopathic constipation - a 4-week, randomized, double-blind, placebo-controlled, dose-response study.
Aliment Pharmacol Ther.
2010; 32(9): 1102–12. PubMed Abstract
| Publisher Full Text
- 112.
Manini ML, Camilleri M, Goldberg M, et al.:
Effects of Velusetrag (TD-5108) on gastrointestinal transit and bowel function in health and pharmacokinetics in health and constipation.
Neurogastroenterol Motil.
2010; 22(1): 42–9, e7–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 113.
Yiannakou Y, Piessevaux H, Bouchoucha M, et al.:
A randomized, double-blind, placebo-controlled, phase 3 trial to evaluate the efficacy, safety, and tolerability of prucalopride in men with chronic constipation.
Am J Gastroenterol.
2015; 110(5): 741–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 114.
Emmanuel A, Cools M, Vandeplassche L, et al.:
Prucalopride improves bowel function and colonic transit time in patients with chronic constipation: an integrated analysis.
Am J Gastroenterol.
2014; 109(6): 887–94. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 115.
Painsipp E, Shahbazian A, Holzer P:
Alosetron, cilansetron and tegaserod modify mesenteric but not colonic blood flow in rats.
Br J Pharmacol.
2009; 158(5): 1210–26. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 116.
Sung KW, Hahn SJ:
Effect of mosapride on Kv4.3 potassium channels expressed in CHO cells.
Naunyn Schmiedebergs Arch Pharmacol.
2013; 386(10): 905–16. PubMed Abstract
| Publisher Full Text
- 117.
Madan K, Ahuja V, Kashyap PC, et al.:
Comparison of efficacy of pantoprazole alone versus pantoprazole plus mosapride in therapy of gastroesophageal reflux disease: a randomized trial.
Dis Esophagus.
2004; 17(4): 274–8. PubMed Abstract
| Publisher Full Text
- 118.
Futagami S, Iwakiri K, Shindo T, et al.:
The prokinetic effect of mosapride citrate combined with omeprazole therapy improves clinical symptoms and gastric emptying in PPI-resistant NERD patients with delayed gastric emptying.
J Gastroenterol.
2010; 45(4): 413–21. PubMed Abstract
| Publisher Full Text
- 119.
Hsu YC, Yang TH, Hsu WL, et al.:
Mosapride as an adjunct to lansoprazole for symptom relief of reflux oesophagitis.
Br J Clin Pharmacol.
2010; 70(2): 171–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 120.
Koshino K, Adachi K, Furuta K, et al.:
Effects of mosapride on esophageal functions and gastroesophageal reflux.
J Gastroenterol Hepatol.
2010; 25(6): 1066–71. PubMed Abstract
| Publisher Full Text
- 121.
Miwa H, Inoue K, Ashida K, et al.:
Randomised clinical trial: efficacy of the addition of a prokinetic, mosapride citrate, to omeprazole in the treatment of patients with non-erosive reflux disease - a double-blind, placebo-controlled study.
Aliment Pharmacol Ther.
2011; 33(3): 323–32. PubMed Abstract
| Publisher Full Text
- 122.
Mugie SM, Korczowski B, Bodi P, et al.:
Prucalopride is no more effective than placebo for children with functional constipation.
Gastroenterology.
2014; 147(6): 1285–95.e1. PubMed Abstract
| Publisher Full Text
- 123.
Piessevaux H, Corazziari E, Rey E, et al.:
A randomized, double-blind, placebo-controlled trial to evaluate the efficacy, safety, and tolerability of long-term treatment with prucalopride.
Neurogastroenterol Motil.
2015; 27(6): 805–15. PubMed Abstract
| Publisher Full Text
- 124.
Cinca R, Chera D, Gruss HJ, et al.:
Randomised clinical trial: macrogol/PEG 3350+electrolytes versus prucalopride in the treatment of chronic constipation -- a comparison in a controlled environment.
Aliment Pharmacol Ther.
2013; 37(9): 876–86. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 125.
Sakurai K, Nagahara A, Inoue K, et al.:
Efficacy of omeprazole, famotidine, mosapride and teprenone in patients with upper gastrointestinal symptoms: an omeprazole-controlled randomized study (J-FOCUS).
BMC Gastroenterol.
2012; 12: 42. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 126.
Sakaguchi M, Takao M, Ohyama Y, et al.:
Comparison of PPIs and H2-receptor antagonists plus prokinetics for dysmotility-like dyspepsia.
World J Gastroenterol.
2012; 18(13): 1517–24. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 127.
Talley NJ, Tack J, Ptak T, et al.:
Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double-blind, placebo-controlled trials.
Gut.
2008; 57(6): 740–6. PubMed Abstract
| Publisher Full Text
- 128.
Ponec RJ, Saunders MD, Kimmey MB:
Neostigmine for the treatment of acute colonic pseudo-obstruction.
N Engl J Med.
1999; 341(3): 137–41. PubMed Abstract
| Publisher Full Text
- 129.
Orenstein SR, Lofton SW, Orenstein DM:
Bethanechol for pediatric gastroesophageal reflux: a prospective, blind, controlled study.
J Pediatr Gastroenterol Nutr.
1986; 5(4): 549–55. PubMed Abstract
- 130.
Parthasarathy G, Ravi K, Camilleri M, et al.:
Effect of neostigmine on gastroduodenal motility in patients with suspected gastrointestinal motility disorders.
Neurogastroenterol Motil.
2015; 27(12): 1736–46. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 131.
Bharucha AE, Low PA, Camilleri M, et al.:
Pilot study of pyridostigmine in constipated patients with autonomic neuropathy.
Clin Auton Res.
2008; 18(4): 194–202. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 132.
Bharucha AE, Low P, Camilleri M, et al.:
A randomised controlled study of the effect of cholinesterase inhibition on colon function in patients with diabetes mellitus and constipation.
Gut.
2013; 62(5): 708–15. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 133.
Broussard JL, Kilkus JM, Delebecque F, et al.:
Elevated ghrelin predicts food intake during experimental sleep restriction.
Obesity (Silver Spring).
2016; 24(1): 132–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 134.
Reichelt AC, Westbrook RF, Morris MJ:
Integration of reward signalling and appetite regulating peptide systems in the control of food-cue responses.
Br J Pharmacol.
2015; 172(22): 5225–38. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 135.
Tack J, Depoortere I, Bisschops R, et al.:
Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis.
Aliment Pharmacol Ther.
2005; 22(9): 847–53. PubMed Abstract
| Publisher Full Text
- 136.
Murray CD, Martin NM, Patterson M, et al.:
Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study.
Gut.
2005; 54(12): 1693–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 137.
Ejskjaer N, Vestergaard ET, Hellström PM, et al.:
Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis.
Aliment Pharmacol Ther.
2009; 29(11): 1179–87. PubMed Abstract
| Publisher Full Text
- 138.
Acosta A, Camilleri M, Busciglio I, et al.:
Short-Term Effects of Relamorelin on Descending Colon Motility in Chronic Constipation: A Randomized, Controlled Trial.
Dig Dis Sci.
2016; 61(3): 852–60. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 139.
Acosta A, Camilleri M, Kolar G, et al.:
Relamorelin Relieves Constipation and Accelerates Colonic Transit in a Phase 2, Placebo-Controlled, Randomized Trial.
Clin Gastroenterol Hepatol.
2015; 13(13): 2312–9.e1. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 140.
Popescu I, Fleshner PR, Pezzullo JC, et al.:
The Ghrelin agonist TZP-101 for management of postoperative ileus after partial colectomy: a randomized, dose-ranging, placebo-controlled clinical trial.
Dis Colon Rectum.
2010; 53(2): 126–34. PubMed Abstract
| Publisher Full Text
- 141.
Shaw M, Pediconi C, McVey D, et al.:
Safety and efficacy of ulimorelin administered postoperatively to accelerate recovery of gastrointestinal motility following partial bowel resection: results of two randomized, placebo-controlled phase 3 trials.
Dis Colon Rectum.
2013; 56(7): 888–97. PubMed Abstract
| Publisher Full Text
- 142.
Ejskjaer N, Dimcevski G, Wo J, et al.:
Safety and efficacy of ghrelin agonist TZP-101 in relieving symptoms in patients with diabetic gastroparesis: a randomized, placebo-controlled study.
Neurogastroenterol Motil.
2010; 22(10): 1069–e281. PubMed Abstract
| Publisher Full Text
- 143.
Wo JM, Ejskjaer N, Hellström PM, et al.:
Randomised clinical trial: ghrelin agonist TZP-101 relieves gastroparesis associated with severe nausea and vomiting--randomised clinical study subset data.
Aliment Pharmacol Ther.
2011; 33(6): 679–88. PubMed Abstract
| Publisher Full Text
- 144.
Ejskjaer N, Wo JM, Esfandyari T, et al.:
A phase 2a, randomized, double-blind 28-day study of TZP-102 a ghrelin receptor agonist for diabetic gastroparesis.
Neurogastroenterol Motil.
2013; 25(2): e140–50. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 145.
McCallum RW, Lembo A, Esfandyari T, et al.:
Phase 2b, randomized, double-blind 12-week studies of TZP-102, a ghrelin receptor agonist for diabetic gastroparesis.
Neurogastroenterol Motil.
2013; 25(11): e705–17. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 146.
Wu C, Chou L, Chen H, et al.:
Effect of fluoxetine on symptoms and gastric dysrhythmia in patients with functional dyspepsia.
Hepatogastroenterology.
2003; 50(49): 278–83. PubMed Abstract
- 147.
Forster J, Damjanov I, Lin Z, et al.:
Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings.
J Gastrointest Surg.
2005; 9(1): 102–8. PubMed Abstract
| Publisher Full Text
- 148.
Chen JD, Schirmer BD, McCallum RW:
Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis.
Am J Physiol.
1994; 266(1 Pt 1): G90–8. PubMed Abstract
- 149.
McCallum RW, Chen JD, Lin Z, et al.:
Gastric pacing improves emptying and symptoms in patients with gastroparesis.
Gastroenterology.
1998; 114(3): 456–61. PubMed Abstract
| Publisher Full Text
- 150.
Lin ZY, McCallum RW, Schirmer BD, et al.:
Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis.
Am J Physiol.
1998; 274(1 Pt 1): G186–91. PubMed Abstract
- 151.
McCallum RW, Dusing RW, Sarosiek I, et al.:
Mechanisms of symptomatic improvement after gastric electrical stimulation in gastroparetic patients.
Neurogastroenterol Motil.
2010; 22(2): 161–7, e50–1. PubMed Abstract
| Publisher Full Text
- 152.
Levinthal DJ, Bielefeldt K:
Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis.
Auton Neurosci.
2016; pii: S1566-0702(16)30033-9. PubMed Abstract
| Publisher Full Text
- 153.
Janssen P, Harris MS, Jones M, et al.:
The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis.
Am J Gastroenterol.
2013; 108(9): 1382–91. PubMed Abstract
| Publisher Full Text
- 154.
van Oudenhove L, Vandenberghe J, Geeraerts B, et al.:
Determinants of symptoms in functional dyspepsia: gastric sensorimotor function, psychosocial factors or somatisation?
Gut.
2008; 57(12): 1666–73. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 155.
Arts J, Caenepeel P, Verbeke K, et al.:
Influence of erythromycin on gastric emptying and meal related symptoms in functional dyspepsia with delayed gastric emptying.
Gut.
2005; 54(4): 455–60. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 156.
Acosta A, Camilleri M, Shin A, et al.:
Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy.
Gastroenterology.
2015; 148(3): 537–546.e4. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 157.
van den Berg MM, Hogan M, Caniano DA, et al.:
Colonic manometry as predictor of cecostomy success in children with defecation disorders.
J Pediatr Surg.
2006; 41(4): 730–6; discussion 730–6. PubMed Abstract
| Publisher Full Text
- 158.
Glia A, Akerlund JE, Lindberg G:
Outcome of colectomy for slow-transit constipation in relation to presence of small-bowel dysmotility.
Dis Colon Rectum.
2004; 47(1): 96–102. PubMed Abstract
| Publisher Full Text
- 159.
Koch KL, Hasler WL, Yates KP, et al.:
Baseline features and differences in 48 week clinical outcomes in patients with gastroparesis and type 1 vs type 2 diabetes.
Neurogastroenterol Motil.
2016; 28(7): 1001–15. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 160.
Bielefeldt K, Raza N, Zickmund SL:
Different faces of gastroparesis.
World J Gastroenterol.
2009; 15(48): 6052–60. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 161.
Spiegel BM, Gralnek IM, Bolus R, et al.:
Clinical determinants of health-related quality of life in patients with irritable bowel syndrome.
Arch Intern Med.
2004; 164(16): 1773–80. PubMed Abstract
| Publisher Full Text
- 162.
Ringström G, Abrahamsson H, Strid H, et al.:
Why do subjects with irritable bowel syndrome seek health care for their symptoms?
Scand J Gastroenterol.
2007; 42(10): 1194–203. PubMed Abstract
| Publisher Full Text
- 163.
Koloski NA, Talley NJ, Boyce PM:
Does psychological distress modulate functional gastrointestinal symptoms and health care seeking? A prospective, community Cohort study.
Am J Gastroenterol.
2003; 98(4): 789–97. PubMed Abstract
| Publisher Full Text
- 164.
O'Brien S, Hyman N, Osler T, et al.:
Sexual abuse: a strong predictor of outcomes after colectomy for slow-transit constipation.
Dis Colon Rectum.
2009; 52(11): 1844–7. PubMed Abstract
| Publisher Full Text
- 165.
Martins ML, Calabresi MF, Quini C, et al.:
Enhancing the versatility of alternate current biosusceptometry (ACB) through the synthesis of a dextrose-modified tracer and a magnetic muco-adhesive cellulose gel.
Mater Sci Eng C Mater Biol Appl.
2015; 48: 80–5. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 166.
Dey N, Wagner VE, Blanton LV, et al.:
Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel.
Cell.
2015; 163(1): 95–107. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 167.
Kashyap PC, Marcobal A, Ursell LK, et al.:
Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice.
Gastroenterology.
2013; 144(5): 967–77. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 168.
Yano JM, Yu K, Donaldson GP, et al.:
Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis.
Cell.
2015; 161(2): 264–76. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 169.
Reigstad CS, Salmonson CE, Rainey JF 3rd, et al.:
Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells.
FASEB J.
2015; 29(4): 1395–403. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 170.
Dimidi E, Christodoulides S, Fragkos KC, et al.:
The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials.
Am J Clin Nutr.
2014; 100(4): 1075–84. PubMed Abstract
| Publisher Full Text
- 171.
van Wunnik BP, Baeten CG, Southwell BR:
Neuromodulation for constipation: sacral and transcutaneous stimulation.
Best Pract Res Clin Gastroenterol.
2011; 25(1): 181–91. PubMed Abstract
| Publisher Full Text
- 172.
Bielefeldt K:
Adverse events of sacral neuromodulation for fecal incontinence reported to the federal drug administration.
World J Gastrointest Pharmacol Ther.
2016; 7(2): 294–305. PubMed Abstract
| Publisher Full Text
| Free Full Text


Comments on this article Comments (0)