Keywords
hepatitis B, hepatitis C, chronic kidney failure, hemodialysis, serological markers
hepatitis B, hepatitis C, chronic kidney failure, hemodialysis, serological markers
Infections with hepatitis B and C viruses (HBV and HCV, respectively) are a major global health problem affecting 240 million people who suffer from chronic HBV infection and about 150 million who suffer from HCV infection1,2. In most cases, these viruses cause chronic infection whose natural course leads to liver cirrhosis, liver failure and/or hepatocarcinoma in affected patients3.
Patients with chronic renal failure (CRF) on hemodialysis are at high risk of contracting viral infections with HBV and HCV, the most common cause of liver disease in these patients4,5. Therefore, strict procedures for the control of hepatitis must be introduced in all dialysis units6.
The geographical distribution of HBV infection is not uniform throughout the world. Depending on the prevalence, different areas are classified as high, intermediate or low endemicity. HBV infection is highly prevalent (8–15%) in Southeast Asia, China, the Philippines, Africa, the Amazon basin and the Middle East. In Eastern Europe, Central Asia, Japan, Israel and Russia the prevalence is intermediate (2.7%), while in North America, Western Europe, Australia and South America the prevalence is low (<2%)7. In Latin America it ranges from 2 to 7%8. In developed countries the prevalence of HBV in patients treated with hemodialysis is 1%9, while in developing countries the prevalence ranges from 2% to 20%4,10,11.
The prevalence of HCV in hemodialysis patients ranges from 2.6 to 22.9% (mean 13.5%) in developed countries, but can reach up to 70% in developing countries12–15. In Latin America, the prevalence of HCV is also highly variable in hemodialysis patients, even within the same country. In Mexico, the prevalence is 6.7%16, in Colombia it ranges from 2.9 to 42.2%17,18, while in Brazil ranges from 6–72% (mean 52%)19.
In recent years there was a significant decrease in the prevalence of both HBV and HCV infections in industrialized countries20. This decline is attributed, among other factors, to the decrease in transfusions, vaccination against HBV and introduction of general biosecurity measures to prevent transmission of infection in hemodialysis units.
The aim of this study was to evaluate the prevalence of HBV and HCV in hemodialysis population dialysis units of four hemodialysis centers in the city of Posadas (Argentina).
In this study, a total of 172 patients diagnosed with CRF under hemodialysis attending four hemodialysis centers of the city of Posadas (Argentina) were included. Ethical approval to conduct the study was obtained by the hemodialysis centres involved in Posada, Argentina: Instituto Privado de Nefrologia srl Roque Saenz Peña I; Instituto Privado de Nefrologia srl Roque Saenz Peña II, Instituto de Nefrologia IOT (Instituto de Ortopedia, Traumatología y Medicina Laboral de alta complejidad); Instituto de Nefrologia Boratti. Institutional Review Board approval from the University of Misiones was not required as the private centers involved are not affiliated with the University and the study was considered a human health research without risks. The protection and control mechanisms for this type of research in Argentina includes inclusion of a written informed consent obtained from each participant".
: Patients who participated in the study went daily to a hemodialysis center and participation was voluntary. Inclusion criteria encompassed individuals with CRF who were just starting hemodialysis treatment 30 days before obtaining blood samples.
All patients signed an informed consent statement for joining the study after explaining the scope of the study. A unique and anonymous code was assigned to each patient and patient confidentiality was ensured.
Blood samples were collected through venipuncture or by finger/heel stick in dry tubes (9.5 ml) with vacuum. After clot retraction, samples were centrifuged at 1,500 rpm for 5 minutes and stored in 5mL aliquots at -20°C (http://www.cdc.gov/measles/lab-tools/serology.html).
Serological markers: surface antigen of hepatitis B virus (HBsAg) and antibodies to hepatitis C virus (antiHCV) were detected by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Wiener, Lab. S.A.I.C.).
The following information from medical records were obtained: age, gender, time on hemodialysis, alanine aminotransferase (ALT) index, history of transfusions and history of drug abuse. An online information system was developed to analyze the information (available at http://bioinf.itiud.org/hemodialysis.php). The system allows computational analysis and deployment of information through generation of reports and statistics charts, designed to provide a simple reading of the results of the study. A descriptive analysis was performed by calculating means, medians and frequencies. Chi-square test was used to analyze the significant relationships between categorical data and Mann–Whitney U test was used for the comparison of continuous data (p<0.005). Data were managed and analyzed using InfoStat software.
Our final sample consisted of 172 patients: 98 males (57%) and 74 females (43%). Their ages ranged from 12 to 85 years with a mean age of 53.4 years (Dataset 1).
All patients had not history of tattooing, piercing, or use illegal drugs, and no human immunodeficiency virus (HIV) coinfection was found in any of the cases.
The cause of CRF in patients on hemodialysis was unknown in 31.4% of the cases, followed by diabetic nephropathy (22.1%). The etiology of CRF for these patients is detailed in Figure 1.
Serum samples from hemodialysis patients were tested for presence of HBsAg and antiHCV by ELISA. Fifteen (15/172) cases were positives for HBsAg, whereas 17 (17/172) cases were positives for antiHCV. Figure 2 shows the distribution of HBsAg and antiHCV cases by age group.
The majority of HBsAg positive cases were females (9/15), but this was not statistically significant (p = 0.16), while of the majority of cases positive for HCV markers were males (14/17); this result was statistically significant (p = 0.02). Figure 3 presents the distribution of HBsAg and antiHCV markers of by gender.

Chi-square Pearson test. HBsAg: p = 0.16; antiHCV: p = 0.02.
All patients participating in this study had 4-hour hemodialysis sessions three times a week and 72.1% of patients showed a mean time of hemodialysis less than 5 years (Figure 4). The duration of hemodialysis was not a significant risk factor for HBsAg (p = 0.9) and antiHCV (p = 0.2) presence.
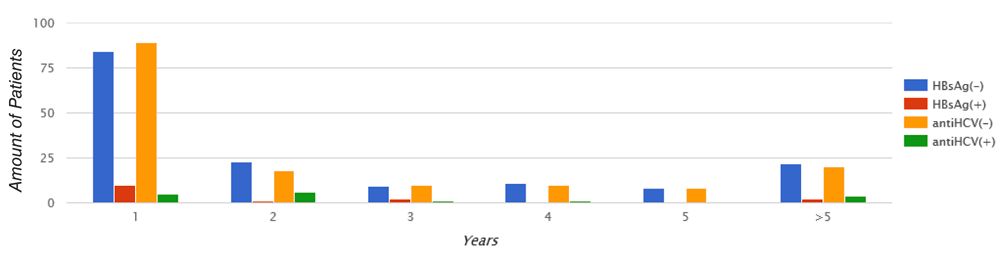
Chi-square Pearson test. HBsAg: p = 0.9; antiHCV: p = 0.2.
Transfusion history was observed in 47% (7/15) of cases positives for HBsAg. Positivity for HBsAg significantly correlated with transfusion (p = 0.003), while 29.4% (5/17) of cases positives for antiHCV did not show correlation with transfusion (p = 0.23) (Figure 5).
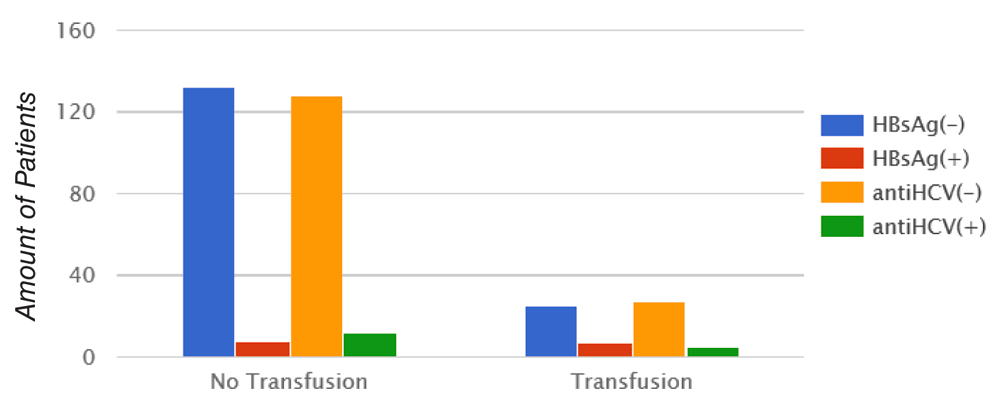
Chi-square Pearson test. HBsAg: p = 0.003; antiHCV: p = 0.23.
In our patient population, 154 (89,5%) cases showed normal concentration of serum ALT level (< 40 U/L), and 18 (10,5%) showed elevation in serum ALT level (>40 IU/L) (Figure 6).
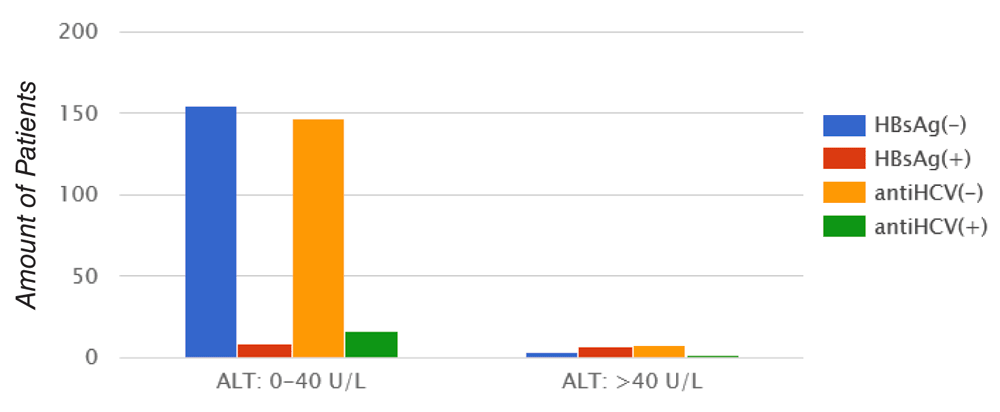
Mann-Whitney U test. HBsAg: p = 0.001; antiHCV: p = 0.002.
A total of six HBsAg positive cases out of 15 (33.3%) showed elevation in serum ALT level, whereas one (5.5%) of the cases positive for HCV out of 17 showed increased serum ALT level. This correlation was statistically significant (p = 0.001 and p = 0.002 respectively).
Patients with CRF on hemodialysis are a group at high risk for HBV and HCV infections.
In Argentina the prevalence of HBsAg varies between the provinces, from 0.17% to 1.79%, and prevalence of antiHCV varies between 1.70% and 21%21. In our study we found a relatively high prevalence of serological markers for HBV and HCV in hemodialysis patients in Posadas (Argentina). The HBsAg antibody (indicator of active HBV infection) was found in 8.7% of the studied cases and antiHCV in 9.9%.
These values are comparable to values reported in several studies on hemodialysis patients. In Peru, HBsAg prevalence ranges from 0.2% to 4.8%22, in Brazil varies between 4% and 28%23,24, in Cuba is 4%25, and in Colombia HBsAg is 22%26.
Various studies in hemodialysis units show different prevalence rates for HCV: in Peru between 90% and 4.65%22, in Uruguay from 16% to 3%27, in Brazil 33.4% to 16%23,28, in Mexico 6.7%16, and in Cuba 90%25. The antiHCV prevalence in dialysis units in Cali (2.9%) and Bogotá (2.7%) is very low17,29, while in Medellin is high (42,2%)18.
By exploring the correlation between risk factors and seropositivity for HBV and HCV in hemodialysis patients, it seemed that timing of hemodialysis was not a risk factor. History of transfusion correlated with the risk of HBV infection, but not with risk of HCV infection. The elevation of ALT enzyme activity was not a good index for HBV and HCV infection in these patients, since normal values were observed in a high percentage of patients positive for HBsAg and antiHCV.
The results of this study demonstrate a high prevalence of serological markers for HBV and HCV infections in CRF patients on hemodialysis in Argentina, compared with results obtained from patients in developed countries.
Therefore, an effective strategy to prevent nosocomial transmission of HBV and HCV in hemodialysis units and reduce the prevalence of infection should be implemented in strict compliance with biosafety standards, measures of education, hygiene and HBV vaccination plans to prevent the infection.
Written informed consent to participate in the study and publish clinical data was obtained by the patients.
F1000Research: Dataset 1. Patient characteristics, 10.5256/f1000research.9068.d13138530
Karina Salvatierra: study design, sample processing, data analysis, discussion of results, and writing the manuscript. Hector Florez: design and analysis of data, drafting the manuscript. Both authors agreed to the final content of the manuscript.
This work has been supported by the Information Technologies Innovation (ITI) Research Group.
The authors would like to thank the hemodialysis patients, authorities, and the staff of the four dialysis centers that collaborated in the study, making this research possible. Both authors agreed to the final content of the article.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
References
1. Delfino CM, Gentile EA, Castillo AI, Cuestas ML, et al.: Hepatitis B virus and hepatitis D virus in blood donors from Argentina: circulation of HBsAg and reverse transcriptase mutants.Arch Virol. 2014; 159 (5): 1109-17 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 1 03 Aug 16 |
read | read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)