Keywords
ABCA4, induced pluripotent stem cells, keratinocytes
ABCA4, induced pluripotent stem cells, keratinocytes
We have demonstrated that a truncated isoform of the retina-specific protein, ABCA4 is expressed in human epidermal keratinocytes. This isoform is required for cell viability at late passage and as such can be used to interrogate the pathophysiology of novel ABCA4 mutations.
ATP-binding cassette, sub-family A, member 4 (ABCA4, previously named ABCR for its retina-specific expression) is a transmembrane protein that is primarily localized to retinal photoreceptors1 where it is responsible for flipping N-retinyldene-phosphatidylethanolamine, a key intermediate in the visual cycle, from the lumen to the cytoplasmic leaflet of photoreceptor outer segment disks1,2. Mutations in ABCA4 cause a build-up of toxic retinoids resulting in a variety of retinal degenerative phenotypes, including Stargardt disease, cone-rod dystrophy and retinitis pigmentosa2. To date over 400 different mutations in ABCA4 have been reported3. Since many of these variants are rare and non-exomic, their pathogenicity is often difficult to prove4. As the primary focus of our group is to develop gene and autologous cell replacement-based treatments for rare inherited retinal degenerative diseases, unambiguous identification of disease-causing mutations is essential.
The neural retina is inaccessible to molecular analysis in living patients. As such, to determine if newly identified mutations in retina-specific genes are disease causing, we use patient-specific induced pluripotent stem cell (iPSCs)-derived retinal neurons5. Typically, iPSCs are generated in our lab using patient-specific keratinocytes isolated from the epidermis of 3 mm punch biopsies. Briefly, the epidermis is separated from the underlying dermis via dispase incubation, and keratinocytes are liberated via trituration. Keratinocytes are subsequently maintained on collagen-coated culture plates in Epilife medium (Gibco; Life Technologies) supplemented with 1% human Keratinocyte Growth Supplement (Gibco). While generating iPSCs from patients suspected of having ABCA4-associated retinal disease, we noticed that primary keratinocyte proliferation was often slow and reprograming efficiencies were low compared to age-matched controls, suggesting that ABCA4 mutations affect keratinocyte physiology directly. In this study a series of western blot, immunocytochemistry, and cytometry experiments were performed to demonstrate that ABCA4 is expressed in human keratinocytes and that ABCA4 mutations alter cellular viability.
To determine if the ABCA4 protein was expressed in keratinocytes, a Western blot comparing lysates isolated from normal human, bovine and murine retina, murine retina with Abca4 deletions, and human keratinocytes was performed. Keratinocytes from three different control individuals of three different ages expressed a truncated protein that corresponded in size to an alternatively spliced 70 kDa ABCA4 isoform (detected previously via rt-PCR4; (Figure 1A)). Immunocytochemical labeling using an antibody targeted against ABCA4 demonstrated a perinuclear pattern of labeling (Figure 1B–C). To determine if mutations in ABCA4 affect cellular proliferation and/or senescence, keratinocytes from three control, three retinal disease patients without ABCA4 mutations, and three patients with molecularly confirmed ABCA4-associated retinal disease were analyzed using propidium iodide and a Tali image based cytometer (Figure 1D). For this analysis, keratinocytes received fresh medium every other day and were passaged routinely once a week. Cell viability readings were assessed at each cell passage. A significant decrease in cell viability was detected between passages 6–9 in cells isolated from patients with ABCA4-associated disease as compared to normal and non-ABCA4 disease controls (Figure 1D). Finally, immunocytochemical staining of normal human skin shows robust ABCA4 expression throughout the keratinizing layer (Figure 1E).
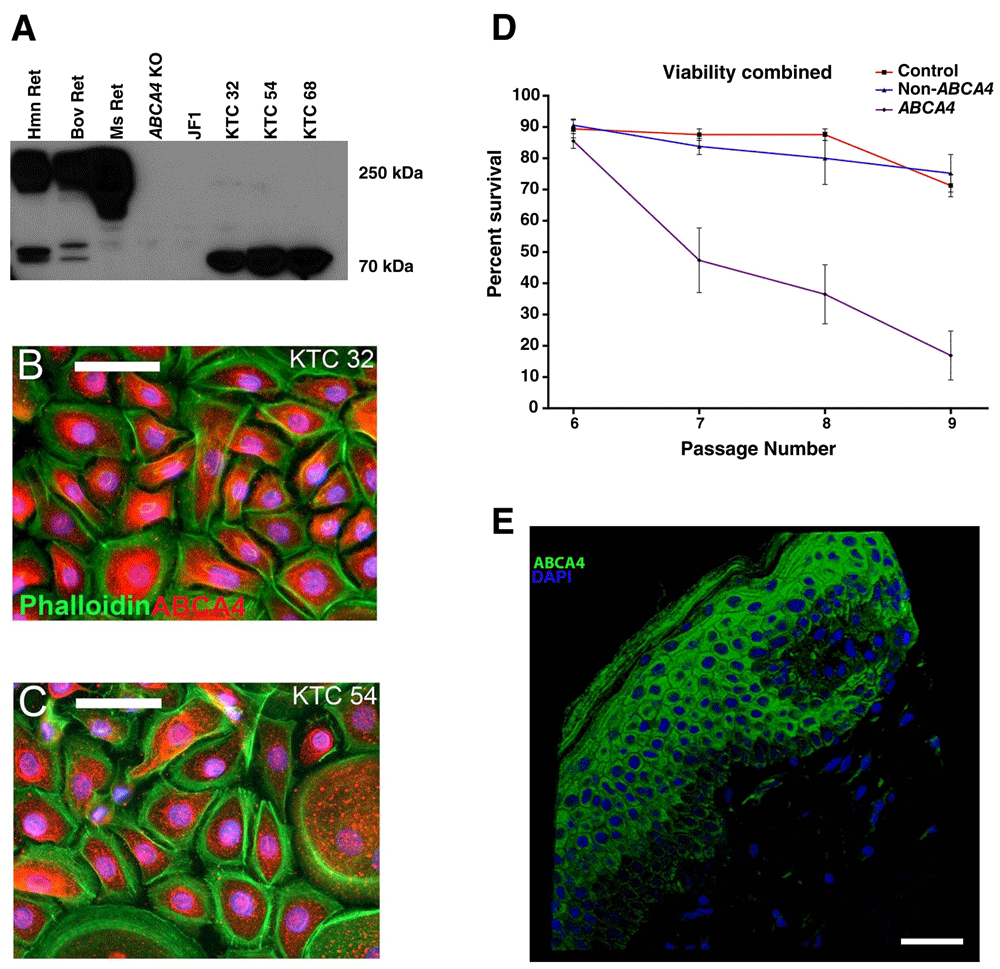
A) Western blot of human (Hmn Ret), bovine (Bov Ret) and murine (Ms Ret) whole retinal lysate, retinal lysate from two mouse strains lacking Abca4 (Abca4 KO and Japanese Fancy (JF1)) and keratinocytes from three individuals 32, 54 and 68 years of age (KTC 32, 54, and 68, respectively). Robust ABCA4 expression is observed at 250 kDa in human, cow and mouse retina, but is completely absent in each Abca4 knockout retina. Keratinocytes from each control individual express a 70 kDa isoform of ABCA4. B–C) Immunocytochemical labeling of control KTC 32 (B) and control KTC 54 (C) patient keratinocytes with an anti-ABCA4 antibody (red) and a filamentous actin stain (Phalloidin; green). A perinuclear pattern of ABCA4 labeling was detected. D) Keratinocyte viability was assessed at passages 6–9 by staining cells with propidium iodide. Keratinocytes assessed from three different individuals with molecularly confirmed mutations in ABCA4 (“ABCA4”) are much less viable compared to control keratinocytes (“control”) and cells from three different patients with non-ABCA4-associated retinal disease (“Non-ABCA4”). E) Immunocytochemical staining of control human skin with the same anti-ABCA4 antibody used in Figure 1A–C above showing ABCA4 labeling (green) throughout the keratinizing layer of the epidermis. Scale bars = 100 μm (B–C) and 40 μm (E).
Taken together, these data demonstrate that a truncated version of the retinal-specific transmembrane enzyme ABCA4 is expressed in epidermal keratinocytes and is required for cell viability at late passage. Although future studies will be needed to elucidate the exact function of ABCA4 in the skin, this finding may be useful for individuals attempting to determine the pathogenicity of novel mutations in the ABCA4 gene.
All patients provided written, informed consent for this study, including skin biospies and use of keratinocytes to generate induced pluripotent stem cells, which was approved by the Institutional Review Board of the University of Iowa (project approval #199904167) and adhered to the tenets set forth in the Declaration of Helsinki.
Western blotting was performed as previously described5–7. Briefly, 40 μg of protein lysate from unaffected human donor retina (Iowa Lions Eye Bank, Coralville, IA), bovine retina (Bud’s Custom Meats Inc., Riverside, IA), control mouse retina (C57Bl/6J; The Jackson Laboratory, Bar Harbor, ME; Cat. No. 00064), Abca4-/- (KO; a kind gift from Dr. Gabriel Travis, University of California Los Angeles), Japanese Fancy mouse retina (a kind gift from Dr. Toshihiko Shiroishi, National Institute of Genetics, Mishima, Japan) and keratinocytes from three individuals ages 32, 54 and 68 were separated via SDS-PAGE on a 4–20% gradient gel, blotted and labeled with a mouse monoclonal anti-ABCA4 antibody at a 1:500 dilution (Santa Cruz Biotechnology, Dallas, TX; Cat. No. sc-65672). Bands were visualized using SuperSignal® West Pico Chemiluminescence Substrate (Thermo Fisher Scientific, Waltham, MA; Cat. No. 34080) and autoradiography. Images of the final blots were captured with an iPhone 6S (Apple, Cupertino, CA).
Keratinocytes were fixed for 10 minutes in 4% paraformaldehyde in 1X phosphate buffered saline (PBS), rinsed in 1X PBS and blocked in immunocytochemical blocking buffer [1X phosphate buffered saline (Cat. No. 10010-023; Thermo Fisher Scientific, Waltham, MA, USA), 3% bovine serum albumin (Cat. No. A30075-100.0; Research Products International Corp., Mount Prospect, IL, USA), 5% normal goat serum (Note: another species can be substituted here to better suit the secondary antibody species; Cat. No. 5425; Cell Signaling, Danvers, MA, USA), 0.5% Triton X-100 (Cat. No. T8787; Sigma-Aldrich, St. Louis, MO, USA), and 0.2% NaN3 (sodium azide; Cat. No. 438456; Sigma-Aldrich, St. Louis, MO, USA)]. Cells were labeled with mouse monoclonal anti-ABCA4 antibody at a dilution of 1:500 (Santa Cruz Biotechnology, Dallas, TX; Cat. No. sc-65672) and stained with Alexa Fluor® 488 Phalloidin at a dilution of 1:1000 (Life Technologies/Thermo Fisher Scientific, Waltham, MA; Cat#: A12379) to label filamentous actin overnight at 4° Celsius. The following morning, cells were washed using wash buffer [1X phosphate buffered saline (Cat. No. 10010-023; Thermo Fisher Scientific, Waltham, MA, USA), 0.2% Tween® 20 (Cat. No. P2287; Sigma-Aldrich, St. Louis, MO, USA)]. ABCA4 was detected using the goat anti-mouse 555 fluorescently conjugated Alexa Fluor® secondary polyclonal antibody at a dilution of 1:1000 (Life Technologies/Thermo Fisher Scientific, Waltham, MA; Cat#: A-21422) for two hours at room temperature. Cell nuclei were coverslipped and counterstained using Poly(vinyl alcohol) (PVA)-based mounting medium containing 1,4-Diazabicyclo[2.2.2]octane (DABCO) [100 μg/ml PVA (Cat. No. 341584; Sigma-Aldrich, St. Louis, MO, USA), 25% v/v glycerol (Cat. No. G9012; Sigma-Aldrich, St. Louis, MO, USA), 0.1M Tris-HCl, pH 8-8.5, 25 μg/ml DABCO (Cat. No. 290734; Sigma-Aldrich, St. Louis, MO, USA) and 4’,6-Diamidino-2-phenylindole dihydrochloride (DAPI) (Cat. No. D9542; Sigma-Aldrich, St. Louis MO, USA; 1:10,000 dilution). Cells were imaged using a Leica DM 2500 SPE confocal microscope (Leica Microsystems, Wetzlar, Germany). Human skin was labeled with anti-ABCA4, secondary antibody and visualized in the same manner.
To determine if mutations in ABCA4 affect cellular proliferation and viability, keratinocytes from three control, three retinal disease patients without ABCA4 mutations, and three patients with molecularly confirmed ABCA4-associated retinal disease were analyzed using propidium iodide (Thermo Fisher Scientific, Waltham, MA; Cat. No. P3566) and a Tali image-based cytometer (Life Technologies, Waltham, MA). Keratinocytes were maintained on collagen-coated culture plates in Epilife medium (Gibco/Life Technologies, Waltham, MA; Cat. No. M-EPI-500-CA) supplemented with 1% Human Keratinocyte Growth Supplement (Gibco/Life Technologies, Waltham, MA; Cat. No. S-001-5). Keratinocytes received fresh medium every other day and were passaged routinely once a week. Cell viability readings were obtained using the Tali image-based cytometer via PI staining and 20 image analysis, i.e. at the time of passage a sample from each cell line was loaded into the Tali, 20 separate images were taken and the number of live vs. dead cells and in turn % viability was automatically generated. This data was collected from cell lines obtained from 7 control individuals, 5 patients with non-ABCA4-associated retinal disease (disease controls) and 13 patients with two molecularly confirmed disease causing ABCA4 mutations. To determine statistical significance, a one-way ANOVA with a Tukey’s post-hoc test was performed use Graphpad Prism software version 6.0b (Graphpad Software Inc., La Jolla, Ca). A p-value < 0.05 was considered significant.
F1000Research: Dataset 1. Raw data for ‘Expression of the retina-specific flippase, ABCA4, in epidermal keratinocytes’, 10.5256/f1000research.8089.d1140778
LAW, RFM, EMS and BAT conceived the study. LAW, EEK and JAP performed the experiments. LAW, RFM, EMS and BAT wrote and approved the final manuscript.
NIH Directors New Innovator Award 1-DP2-OD007483-01 (BAT); NEI EY016822 (EMS); NEI EY024605 (RFM); Research to Prevent Blindness; Stephen A. Wynn Foundation.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 18 Feb 16 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)