Keywords
inner hair cell, synaptic ribbon, otoferlin,
inner hair cell, synaptic ribbon, otoferlin,
In the mammalian cochlea, inner hair cells (IHCs)—the genuine sensory cells of the cochlea transform sound-induced mechanical signals into a neural code at their ribbon synapses. Upon hair bundle deflection, mechanotransducer channels, located in the stereociliar tips, provide hair cell depolarization. This process leads to presynaptic glutamate release from IHCs onto spiral ganglion neurons (SGNs), and ultimately activates the auditory pathway. Coding of sound at IHC ribbon synapses achieves impressive performance: each glutamatergic presynaptic active zone (AZ) of an IHC provides the sole excitatory input to a postsynaptic SGN. Yet, each single AZ drives SGN spike rates at sound onset in the kilohertz range and supports firing at hundreds of hertz during ongoing stimulation. Moreover, these synapses are capable of transmitting information on the timing of the stimulus with submillisecond precision.
The underlying mechanisms that mediate this performance have remained enigmatic but likely relate to the molecular and structural specializations of the IHC ribbon-type AZ. Here, we briefly review the latest progress on the molecular anatomy and physiology of the IHC ribbon synapse, with a focus on the presynaptic AZ (for recent reviews of the postsynaptic SGN, see 1,2.) Dysfunction or loss of IHC synapses causes a specific form of sensorineural hearing impairment: auditory synaptopathy (recently reviewed in 3). We will then summarize recent experimental and theoretical work that has corroborated the Ca2+ nanodomain hypothesis of Ca2+ influx-exocytosis coupling at IHC ribbon synapses. Finally, we will provide a brief overview of the current state of the debate on the mode of exocytosis at the hair cell AZ, which remains a hot topic of current research.
The synaptic ribbon represents the most prominent structural deviation from “conventional” glutamatergic synapses of the vertebrate central nervous system (Figure 1). Depending on the cell type, developmental stage, and animal species under investigation, the ribbon can assume various shapes and sizes4–6. The main structural component of synaptic ribbons is RIBEYE7, a protein assembled from an aggregation-prone A domain and an enzymatically active B domain, both of which are transcribed from the CtBP2 gene8. The synaptic ribbons help cluster large complements of Ca2+ channels and readily releasable vesicles at the IHC AZ, thereby enabling synchronous auditory signaling and also promoting continuous vesicle replenishment9–11. Ribbons are also critical for sensory processing in the retina, where they serve similar functions12–14, and, in addition, seem to play a role in coupling Ca2+ channels to release sites15.
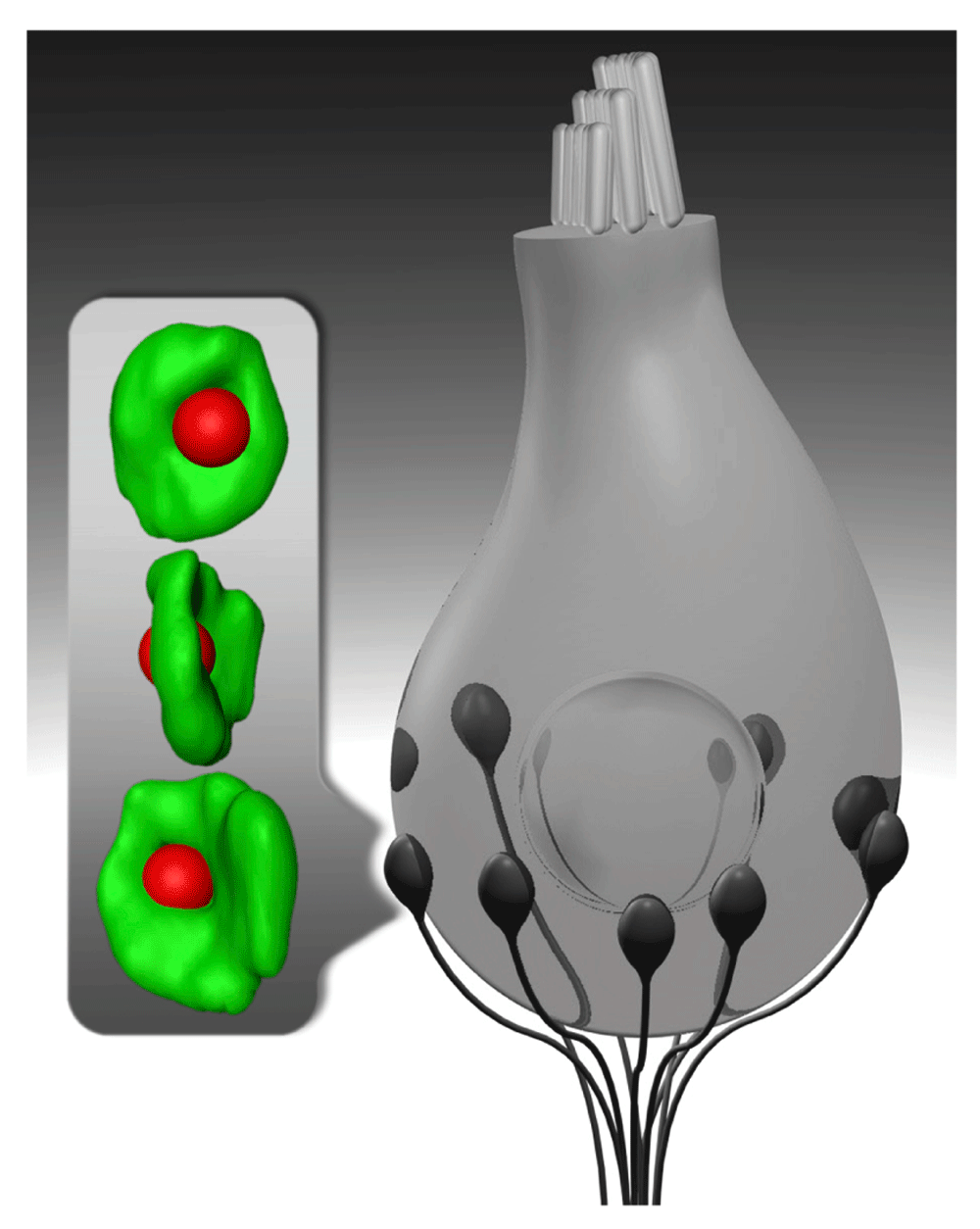
Schematic drawing of an inner hair cell (gray) and its synapses with spiral ganglion neurons (black). Inset shows super-resolution (4Pi) images of an immunolabeled inner hair cell synapse with the synaptic ribbon (red) placed opposite to the center of the postsynaptic AMPA receptor cluster (green). Each spiral ganglion neuron is thought to receive input from one ribbon-type inner hair cell active zone at the postsynaptic swelling of its peripheral neurite.
In addition to unexpectedly finding that IHC ribbon synapses appear to operate without neuronal SNAREs16 and the classic neuronal Ca2+ sensors synaptotagmin 1 and 217,18, we have recently come to realize that SNARE regulators such as complexins19,20, as well as priming factors of the Munc13 and CAPS families21 which are critical for transmission at many synapses, also seem to be missing from IHCs. Instead, hair cells employ the multi-C2-domain protein otoferlin22, a member of the ferlin family of membrane fusion-related proteins (reviewed in 23,24), which is a tail-anchored protein and requires the TRC40 pathway for efficient targeting to the endoplasmic reticulum25. Otoferlin clusters below the synaptic ribbon21 and seems to assume multiple roles in hair cell exocytosis. For example, otoferlin has been suggested (i) to act as the putative Ca2+ sensor in IHCs22,26, (ii) to facilitate vesicular priming and replenishment21,27, and (iii) to participate in exocytosis-endocytosis coupling through direct interaction with the adaptor protein 2 (AP-2) complex28,29. It is tempting to speculate that IHCs evolved this unconventional molecular machinery in order to achieve the utmost performance. One possible requirement could be a rapid and low-affinity engagement of synaptic vesicles with release sites with molecular links to a nearby Ca2+ channel, followed by rapid clearance of vesicular lipid and proteins from that site for it to be quickly reloaded. Clearly, more work is required to elucidate the molecular fusion machinery of IHCs.
Besides the presence of otoferlin, IHC AZs are characterized by large Ca2+ channel clusters, which localize underneath the presynaptic density30 and consist predominantly of pore-forming L-type CaV1.3 subunits31,32, auxiliary CaVβ233, and likely yet-to-be-identified CaVα2δ subunits34. Ca2+ channel clustering depends on multiple molecular scaffolds, such as Bassoon or the ribbon (or both)10,35 as well as RIM2α and β36. The seamless interplay and correct localization of these proteins is not only required for establishing a normal Ca2+ channel complement10,36 but also critical to stabilize a large readily releasable pool of vesicles at the AZ10,11,36. Moreover, IHC AZs contain additional scaffolds such as Piccolino, a short splice variant of Piccolo37, and the Usher protein harmonin that directly interacts with presynaptic Ca2+ channels, regulates their gating, and likely promotes their proteasomal degradation38,39. Although the endocytic machinery is still largely uncharted, it was recently shown to include AP-228,29, dynamins40,41, amphiphysin, and clathrin heavy chain41.
The manner in which Ca2+ influx couples to vesicle fusion critically determines the properties of synaptic transmission. Two limiting cases can be distinguished (reviewed recently in 42–44): (i) “pure” Ca2+ nanodomain control, in which the Ca2+ driving the fusion of a given vesicle is contributed by an individual voltage-gated Ca2+ channel, and (ii) “pure” Ca2+ microdomain control, where the amount of Ca2+ at the Ca2+ sensor is governed by a population of Ca2+ channels, with negligible impact of individual channels. Aside from the precise topography of the channels with respect to the vesicular Ca2+ sensors and their numbers and open probabilities, the Ca2+-binding properties of the vesicular Ca2+ sensor and the cytosolic Ca2+ buffering at the AZ govern the coupling45. Previous work has examined the Ca2+-binding properties of the Ca2+ sensor of fusion by combining whole-cell Ca2+ uncaging and membrane capacitance measurements in IHCs of mice right after hearing onset46. In these experiments, a requirement for four to five Ca2+ ions to bind cooperatively prior to fusion was indicated, and an overall low Ca2+ affinity of the sensor can be assumed. Note however, that this approach yielded massive exocytosis (added membrane equivalent to 15% of the cell’s surface). Hence, it is unlikely to be entirely mediated by exocytosis at IHC AZs, but probably also involved extrasynaptic exocytosis. This calls for revisiting the intrinsic Ca2+ dependence of synaptic vesicle fusion by using refined approaches to exocytosis at AZs, ideally of more mature IHCs.
Classic47 and more recent30,31,48 work indicates that hair cell AZs harbor tens of Ca2+ channels on average. However, within a given IHC, regardless of its tonotopic position, the number of Ca2+ channels per AZ varies dramatically. This presynaptic heterogeneity is thought to be related to the requirements of wide dynamic range sound encoding30,49–51. Interestingly, IHCs display opposing gradients of their AZs for Ca2+-channel complement (higher at the modiolar side, facing the ganglion) and voltage-dependence of activation (voltage for half-maximal activation more hyperpolarized at the pillar side, facing away from the ganglion)52.
Moreover, depending on the experimental strategy, estimates for the maximal Ca2+ channel open probability at IHC AZs vary between 0.248 and 0.430. To date, the exact topography of individual Ca2+ channels within the observed stripe-like clusters beneath the ribbon and their putative molecular linkers to vesicular release sites remains to be experimentally determined. Here, biophysically constrained modeling has proven to be a useful tool in exploring the consequences and feasibility of various scenarios (see below, 30,53). Moreover, another interesting aspect in this context will be the detailed identification of the molecular processes governing AZ maturation, in particular, in regard to the progressive tightening of Ca2+ channel-synaptic vesicle coupling during early postnatal development30,48,54.
Endogenous Ca2+ buffering has been studied in hair cells of various species53,55–58 typically revealing substantial concentrations of Ca2+-binding sites (up to a few millimolar). Recently, measurements of exocytic membrane capacitance changes in mutant IHCs lacking the three major cytosolic EF-hand Ca2+-binding proteins (that is, calbindin-28k, calretinin, and parvalbumin) were combined with Ca2+ buffer substitution—using different concentrations of synthetic Ca2+ chelators with either slow (EGTA) or fast (BAPTA) kinetics—and computational modeling of stimulus-secretion coupling53. With this combinatorial approach, the effective average coupling distance between the Ca2+ sensor of the release site and the nearest Ca2+ channels was estimated to equate to approximately 17 nm in mature IHCs and this is well in line with a Ca2+ nanodomain-like control of exocytosis and similar to previous estimates (approximately 23 nm;59).
The notion of a Ca2+ nanodomain-like control of exocytosis is supported by observations of a lower apparent Ca2+ cooperativity (approximately 1.4) of IHC exocytosis upon changes in the channel open probability, when compared with that found with changes in single-channel Ca2+ current (3–4;30,31,59). The interpretation of these discrepant apparent cooperativity estimates as evidence for Ca2+ nanodomain-like control of exocytosis was further substantiated by modeling30. There, 50 Ca2+ channels were distributed over an area of 80 × 400 nm, aiming to match the Ca2+-channel clusters, assuming different topographies of the Ca2+ channels to a dozen release sites that were placed at the rim of the Ca2+ channel cluster. Modeling of Ca2+-triggered exocytosis was constrained by experimental observations as much as possible. When channels were randomly positioned, the apparent Ca2+ cooperativity of exocytosis during changes in the number of Ca2+ channels was close to two, and hence higher than the experimentally observed value of 1.4. The physiological Ca2+ cooperativity was best matched when allocating one molecularly coupled channel to each release site, while the other channels were distributed randomly but respected an exclusion zone of 10 nm around the coupled channels. This was taken to support the notion of Ca2+ nanodomain-like control of exocytosis at the IHC AZs of mice after hearing onset, likely realized by molecular coupling of a “private” channel to the release site.
During development, Ca2+ influx-exocytosis coupling tightens from Ca2+ microdomain-like control to Ca2+ nanodomain-like control30, as also found for other synapses60. At this point, Ca2+ microdomain control of exocytosis seems less likely for mature IHC synapses. Initially, this mechanism had been considered as an explanation for the low apparent Ca2+ cooperativity of exocytosis in mature IHCs, which was thought to employ a linear Ca2+ sensor (one Ca2+-binding step by synaptotagmin 4) after the onset of hearing61. Alternatively, a lower cooperativity, even in the presence of a sensor with several Ca2+-binding steps, was suggested to result in a linear apparent Ca2+ dependence in whole-cell membrane capacitance measurements, due to summing exocytosis from heterogeneous, but Ca2+ microdomain-governed AZs in IHC capacitance measurements of exocytosis62. However, whereas the former hypothesis seems hard to reconcile with the comparable and supralinear intrinsic Ca2+ dependence of fusion prior and after hearing onset30, the latter appears incompatible with the indications for Ca2+ nanodomain control of exocytosis at the single-synapse level59.
Future experiments should elucidate the topography and mobility of individual Ca2+ channels within the cluster and dissect putative linker proteins connecting release sites to the nearest Ca2+ channel(s). Moreover, further characterization of the molecular composition of the CaV1.3 Ca2+ channel complexes, also testing the presence and role of splice variants of the pore-forming CaV1.3α subunit, will assist in understanding (i) the molecular mechanisms that govern the heterogeneity of Ca2+ channel expression, (ii) the developmental tightening of excitation-secretion coupling, and (iii) the respective contributions of individual Ca2+ channel variants to shaping the efficiency of presynaptic Ca2+ influx at individual IHC AZs.
We will now focus on the mode of vesicular release at auditory ribbon synapses, a mechanism that remains only partially understood. The phenomena that raised uncertainty about this fundamental process of synaptic transmission are (i) a remarkable heterogeneity of AMPA receptor-mediated excitatory postsynaptic current (EPSC) amplitudes, that can range from about 20 to more than 800 pA at postsynaptic SGN terminals of rodents and (ii) the differences in release kinetics, as reflected in variable rise times and waveforms of the EPSCs63.
Conventionally, quanta of neurotransmitter are released spontaneously (“uniquantal release”), thereby producing so-called miniature EPSCs (mEPSCs). These mEPSCs are uniform in size and have characteristic monoexponentially decaying waveforms. Such mEPSCs are thought to correspond to spontaneous and statistically independent fusion of individual vesicles, constituting the basis of the quantal hypothesis of transmitter release64. At IHC synapses, large variance of EPSC amplitudes and waveforms is found in individual postsynaptic boutons even in complete absence of stimulation. Synchronized (statistically dependent) release of multiple vesicles (“synchronized multiquantal release”) was offered as a plausible mechanism explaining such EPSC heterogeneity59,63,65. Synchronized multiquantal release has also been indicated for hair cell synapses of turtle and bullfrog papillae66–69, and for other sensory synapses, such as those in retinal bipolar cells70–72.
Heterogeneity of the EPSC shape might result from varying degrees of synchronicity of multiquantal release63,73. Potential mechanisms mediating synchronized multiquantal release include release site synchronization and compound or cumulative fusion of vesicles (reviewed in 2,4,74). The synaptic ribbon might contribute to synchronizing multiquantal release by clustering presynaptic Ca2+ channels and tethering a large complement of release-ready synaptic vesicles10,21,36,71,75. One thought is that Ca2+ influx through an individual Ca2+ channel could synchronously trigger the fusion of several nearby vesicles underneath the synaptic ribbon66. Alternatively, vesicles might pre-fuse to each other to form larger quanta prior to fusion to the plasma membrane (compound exocytosis), or fuse to a vesicle that is in the process of release (cumulative exocytosis).
Recently, uniquantal release through a dynamic fusion pore has been suggested as an alternative hypothesis for IHC synapses of rodents76. The motivation came from considering the spike rates of hundreds of hertz over prolonged periods of time, which, according to the multiquantal hypothesis (on average, six vesicles per EPSC59), would require at least sixfold-higher vesicle release rates, which seem very high considering measured rates of sustained exocytosis of about 700 vesicles per second27. Moreover, in conditions omitting the synchronizing effect of presynaptic Ca2+ entry, large monophasic, as well as multiphasic, EPSCs persisted76, seemingly arguing against a Ca2+-synchronized multiquantal release scenario at the IHC ribbon synapse. In addition, biophysically constrained mathematical modeling of compound exocytosis suggested the presence of large vesicles near the AZ membrane, which was not found in electron microscopy of stimulated samples. These latter findings are difficult to reconcile with a synchronized multiquantal release mode to take place at mammalian IHC ribbon synapses. So, could a uniquantal release model offer an explanation for the large heterogeneity of EPSC amplitudes?
Uniquantal release through a dynamic fusion pore has also been proposed for other synapses77,78; however, for this scenario to be plausible at IHC synapses, two major questions arise: (i) could the glutamate content of a single synaptic vesicle elicit the observed large EPSCs (on average, about 300 pA) and (ii) could transmitter release be governed by a fusion pore that might regulate the extent and timing of release events? Using mathematical modeling—constrained by morphological estimates of the postsynaptic AMPA receptor clusters (Figure 1) and assumptions on glutamate content and AMPA receptor density and function—the study concluded that single-vesicle release might suffice to trigger large-amplitude EPSCs76. Moreover, model prediction and deconvolution of EPSCs suggested that fusion pore regulation could account for the observed variable EPSC shapes. In detail, the authors suggested a model in which there is either immediate and full collapse upon vesicle fusion (potentially explaining monophasic EPSCs) or alternatively the formation of a transitory instable fusion pore prior to collapse, potentially flickering open and closed—a mechanism permitting progressive glutamate unloading that may explain the observation of multiphasic EPSCs. In addition, variable vesicle size that occurs also in the absence of homotypic vesicle-to-vesicle fusion and different filling states of vesicles79 might contribute to the EPSC heterogeneity at IHC synapses. Finally, other mechanisms such as spill-over of glutamate from neighboring synapses and postsynaptic receptor properties need to be considered when relying on postsynaptic recordings.
These different hypotheses might not be mutually exclusive, and appear to strongly depend on the chosen experimental model system. Further experiments, such as (i) membrane capacitance recordings of individual fusion events, (ii) electron tomography of synapses immobilized within milliseconds after stimulation, or (iii) super-resolution fluorescence live-cell imaging of vesicular exocytosis, will help in the future to pinpoint the mode of exocytosis at hair cell ribbon synapses.
This work was supported by grants from the German Research Foundation through the Collaborative Research Center 889 and the Leibniz Program (to TM).
We thank Darina Khimich and Alexander Egner for contributing the 4Pi image and Hartmut Sebesse for the artwork.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 26 Aug 16 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)