Keywords
Capillaries, Microcirculation, Altitude, Microscopy, Oxygen
This article is included in the University College London collection.
Capillaries, Microcirculation, Altitude, Microscopy, Oxygen
EBC Everest Base Camp
FOV Field of view area
[Hb] Haemoglobin concentration
Hct Haematocrit
IDF Incident Dark Field
KTM Kathmandu
LON London
SpO2 Peripheral oxygen saturation
The physiological processes involved in acclimatisation to high altitude attempt to maintain adequate oxygen delivery as the partial pressure of oxygen decreases. Traditionally, research has concentrated on global haemodynamics and the macrocirculation, variables such as cardiac output1, oxygen saturations2 and haemoglobin concentration [Hb]3. Far fewer studies have focused on the microcirculation, which regulates blood flow to match micro-regional oxygen demand. Disruption of microvascular blood flow could explain a failure of acclimatisation in some individuals as well as the well-documented exercise limitation that occurs at altitude despite normalisation of systemic oxygen delivery4. The precise role of the microcirculation in acclimatisation to hypoxia, however, remains unclear.
Teleological reasoning would suggest that increasing capillary density could provide a means to augment oxygen flux and tissue oxygenation through a reduction in the inter-capillary distance5. Whilst plausible, data on this theory remains contradictory, though this may in part relate to the dissimilar tissues observed. In human skeletal muscle biopsy samples previously exposed to hypobaric hypoxia, no evidence of neovascularisation has been demonstrated6–9. Interestingly, in each instance whereby the capillary density was initially thought to increase, no change in the capillary-to-fibre ratio was observed. The perceived rise in capillary density were therefore interpreted as being secondary occurrences in response to a reduction in skeletal muscle mass. Conversely, an increase in the density of sublingual microcirculatory vessels to >25 μm was demonstrated on ascent to high altitude10,11, a response that was further amplified after prolonged exposure to hypoxia10. In this instance, what remains to be determined is whether the observed changes in vessel density are due to microvascular recruitment secondary to increased blood viscosity (and thus quickly reversible), or neovascularization (which is likely to be sustained). Moreover, the question of what happens to vessel density following re-exposure to normoxia remains to be elucidated.
We therefore piloted a novel modification of a previously described technique for calculating changes in capillary density12,13 on ten individuals, to see if we could firstly support or refute previous findings on ascent to high altitude, and secondly see if the changes observed persist on descent. Additionally we monitored haemoglobin concentration and haematocrit data to see if we could support of refute mechanisms of altered density relating to vessel recruitment.
The study was undertaken as part of the Xtreme Everest 2 research expedition (XE2)14. The study design, risk management plan and protocol were approved (in accordance with the declaration of Helsinki) both by the University College London Committee and the Ethics of Non-National Health Service Human Research, and the Nepal Health Research Council (Reg no. 139/2012). Written consent was obtained from all participants. Baseline images of the labial capillaries were initially obtained from ten individuals in London (LON) (35m) in December 2012 and January 2013. Sequential images were taken after an 11 day ascent to Everest Base Camp (EBC-early) (5300m), then after 6 weeks residence at Everest Base Camp (EBC-late), and finally on descent, over 5 days, to Kathmandu (KTM) (1300m) in May 2013.
Images were obtained using a CytoCam-IDF video microscope (Braedius, Medical BV, Netherlands). This new device is based on the principle of Incident Dark Field (IDF) imaging, which uses polarized green light (wavelength 548nm) produced from LEDs to visualize, in real time, the sublingual microvasculature. Its high resolution imaging sensor (14 Mpixel) allows for a 50% increase in optical resolution (300 lines/mm) compared to previous Sidestream Dark Field imaging devices, and it generates a far larger field of view. With the participant lying in the supine position having rested for a minimum of 10 minutes, the CytoCam-IDF device’s probe was introduced into their mouth and placed on the mucosal surface of the inner lip. Once a suitable image was visualised on the screen of the CytoCam-IDF monitor, (Figure 1), 1 second of digital video footage was recorded. This process was conducted on all four lip quadrants (right upper lip, left upper lip, right lower lip, left lower lip), and at each quadrant four separate videos were acquired. Two trained investigators (EGK, PH) obtained all the data.
To determine capillary density, analysis was conducted offline by two independent investigators blinded to the participant identity, the testing conditions and the imaging site (EGK, JC). Using the company’s own video software (CytoCamTools V1, Braedius, Netherlands), a single still frame was projected on the computer screen and the number of capillary loops per image frame was counted manually. Partly visualised capillaries were included if the observer was assured that the vessel was a capillary due to its morphology. Subsequently, the mean capillary density was calculated from the four images obtained in each lip quadrant, and from these four results, the mean total lip density obtained. Capillary density was defined as the number of capillaries counted per field of view area (FOV), which equates to 1.79 mm2. The haemoglobin concentration ([Hb]) (Hemocue AB, Hemocue, Sweden) and haematocrit (Hct) (Sigma 1–14 microcentrifuge, Sigma, Germany) were obtained from whole blood samples, and peripheral arterial oxygen saturation (SpO2) measured (Nonin Onyx 9500, Nonin Medical Inc, Minnesota, USA) on the same days as microcirculatory imaging was performed.
As data were not normally distributed, they were described by median and interquartile range. Repeated sets of paired values were compared using Kruskall Wallis ANOVA, whilst comparisons of values between LON baseline and other sites was by Wilcoxon Signed Rank Test. Correlation between different variables was performed using Spearman’s rank correlation coefficient, and concordance between analysing investigators using intra-class correlation coefficient. All statistical analysis was undertaken on SPSS version 21 (SPSS Inc., Chicago, IL, USA), and a P value of <0.05 was taken to indicate statistical significance.
CytoCam-IDF imaging was conducted on all ten individuals on the first two occasions, however only eight individuals had data captured on descent. No problems were encountered with the device or image acquisition. Mean laboratory barometric pressure and mean temperature for each location is shown in Table 1.
| Site | Atmospheric Pressure (kPa) | Temperature (°C) | Humidity (%) |
|---|---|---|---|
| London | 100.6 (0.2) | 16.9 (1.8) | 35.4 (6.5) |
| Everest Base Camp | 53.0 (0.2) | 12.9 (8.2) | 37.8 (17.5) |
| Kathmandu | 86.8 (0.4) | 23.8 (3.4) | 47.4 (15.7) |
Changes in labial capillary density are shown in Figure 2. Compared with LON (median 22.8 capillaries per field of view area (20.7–26.8)), capillary density was significantly increased at EBC-early 25.3 (24.5–30.6; p=0.021), EBC-late 32.5, (28.4–36.63; p=0.012), and on descent in KTM 31.0 (24.0–35.13; P=0.017). Between EBC-early and EBC-late, capillary density increased significantly (p=0.017), however there was no significant decline in density between EBC-late and KTM (p = 0.069).
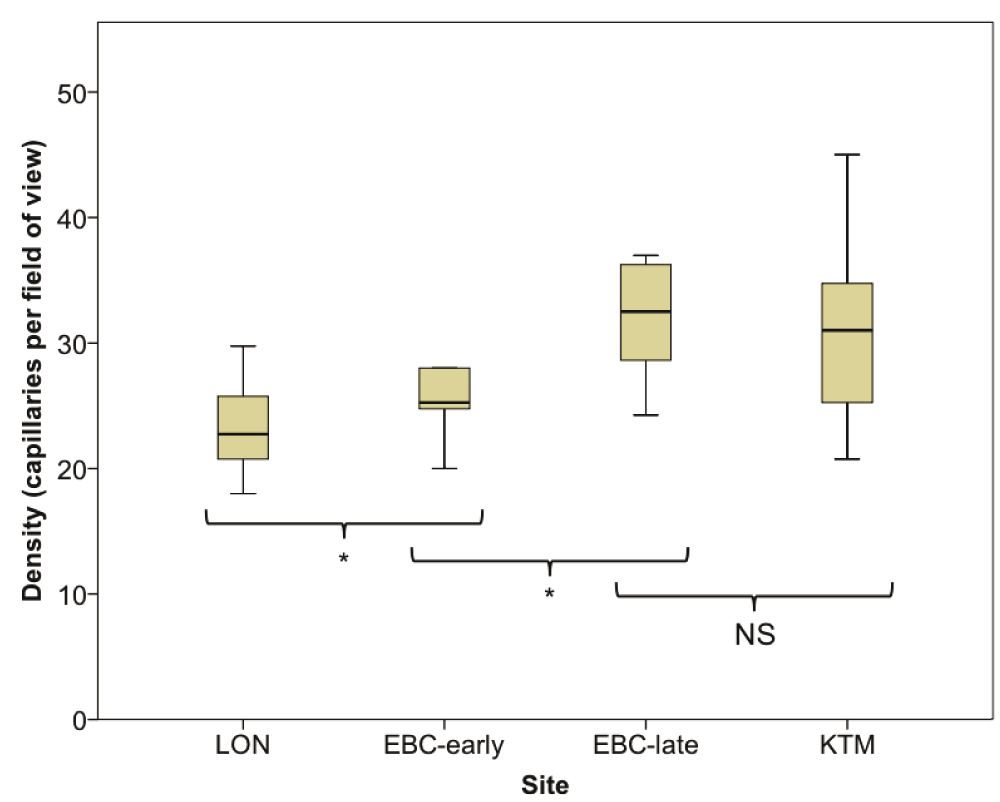
Changes in [Hb], Hct and SpO2 at each site are shown in Table 2. There was a significant increase in [Hb] between LON and EBC-early (p=0.007), and EBC-early and EBC-late (p=0.011), and a decrease between EBC-late and KTM (p=0.008). There was also a significant increase in Hct between LON and EBC-early (p=0.007), and EBC-late and KTM (p=0.012), but no significant change between EBC-early and EBC-late (p=0.191). Between the sites on ascent, the increase in vessel density demonstrated an inverse relationship with the SpO2, however at each altitude there was no correlation between vessel density and [Hb], Hct or SpO2.
To assess whether an image capture time of 1 second was indicative of that captured over longer periods of time, we obtained 30 seconds of footage from four individuals at two different locations. From this we randomly selected one frame per five seconds of footage, and counted the number of capillaries per field of view area. The values of these may be seen in Table 3, as too can the mean and standard deviations for each set of frames, the latter of which demonstrates a highest value of only 0.52 capillaries per field of view area.
This study demonstrates for the first time, persistence of in vivo sublingual microvascular density increase on re-exposure to normoxia after a prolonged period of hypobaric hypoxia at high altitude. We utilised an infrequently used imaging and analysis technique that we had purposefully adapted to suit our needs, and found our data aligned with previously published work on blood vessel density at altitude10.
Using the data obtained from corresponding blood samples, it is possible to speculate on the adaptive processes occurring at each measurement point. As previously described, we observed a significant rise in [Hb] (14.5 g/dl to 16.4g/dl; p=0.007) and Hct (44% to 52%; p=007)) on ascent to altitude. Whilst this polycythaemia increases arterial oxygen content, blood viscosity also rises, altering its rheology. Under normal physiological conditions, a considerable proportion of the microcirculation is thought to be ‘unrecruited’, acting as a reservoir for times of increased metabolic needs15. As Hct rises, these reserve vessels are recruited, and microvascular density increases, along with functional capillary density15–18. Thus a secondary benefit to increased Hct is achieved; a reduction in the diffusion distance from capillaries to mitochondria. Importantly however, it should be noted that in normal capillary Hct is generally 50% less than systemic Hct owing to the streamlined blood flow in narrow capillaries19. The effect of hypoxia on this association is unknown. Whether or not neovascularisation had occurred on arrival at high altitude is difficult to say, although due to the short time between measurement points the chances of this being the case are low20.
After 6 weeks spent at altitude, a further, and far greater, increase in vessel density was apparent; EBC-early 25.3 capillaries per field of view area, EBC-late 32.5 (p=0.017). Over the same time period, [Hb] had significantly risen, whilst Hct had not. As Hct is a more reliable indicator of viscosity between the two variables21, it seems unlikely that further recruitment of the microvasculature had occurred, yet it is plausible that neovascularisation had. Increased levels of vascular endothelial growth factor (VEGF) have been detected in subjects ascending to high altitude22; its role in angiogenesis perhaps explaining the observed rise in microvascular density10. Such adaptations lead to improved tissue oxygenation by a reduction of the inter-capillary distance, whilst maintaining a sufficiently low, and thus fluid Hct to permit flow of red blood cells in the microvasculature.
On descent to a lower altitude (KTM) there was no significant fall in microvascular density when compared to EBC-late (p = 0.069), however, a much greater number of vessels (36% increase) was evident when compared with baseline testing in LON. Whilst vessel density was thus unaltered on descent, over the same time point [Hb] and Hct values significantly declined (p=0.012). When compared to the original LON values, Hct on descent to KTM was significantly higher (44.0% and 50.0% respectively (p=0.011)), however, [Hb] was not (14.5 and 16.0g/dl (p=0.052)). Teasing apart the relative contributions of vessel recruitment and neovascularisation to the observed changes in sublingual microcirculatory density is challenging. Whilst the failure of vessel density to return to baseline after descent suggests some neovascularisation, neither [Hb] nor Hct had normalised at the time of the final readings so a raised blood viscosity could perhaps be maintaining a heightened level of capillary recruitment. A combination of the two processes would make sense as continually increasing [Hb] to improve oxygen delivery would eventually be counter productive. Indeed in Tibetans, who have been exposed to environmental hypoxia for many generations, there is a clear reduction in [Hb] compared to populations who have been exposed to these conditions for less time23–26. This suggests Tibetans utilize alternative long-term strategies for chronic adaptation to hypobaric hypoxia, ones that do not rely on maintaining a high [Hb]. It is plausible that one such means would be to increase their capillary density.
The use of the described methodology was also novel. A similar technique has been used twice previously; once in the assessment of coronary artery disease in diabetes13 and the other in a study investigating hypertension and rarefaction during treatment with Telatinib12. In these instances, data capture involved recording sublingual images for 1 minute13 or 30 seconds12 per quadrant, however we altered this time period by using an extremely short capture phase for data acquisition (< 1 second). Crucially, this allowed us to readily obtain snap shot images to reveal data about microvasculature density, whilst avoiding concerns surrounding probe and patient movement, in addition to issues relating to pressure artefact. Analysis was rapid, simple and reproducible; in this study it had an observer mean intra-class correlation coefficient of 0.91 (95% CI 0.84 – 0.96). Previously no difference in capillary density was observed in ten individuals between lip quadrants, and the reproducibility of the technique to determine capillary density was moderate to high with a coefficient of variation of 4.6%12. Of note, the technique does not allow assessment of microvascular flow, nor does it yield information on heterogeneity of microvascular blood flow, however, we propose it to be a robust method for the assessment of labial vessel density that could be conducted after only a short user training period.
The small number of participants used in this study could be considered a study limitation. As we were both employing a newly-adapted data acquisition technique, and using a novel device at altitude, no power calculation was performed. This therefore increases the risk of a type 2 error. Other limiting factors include the environmental considerations associated with high altitude research in a remote field environment. These include fluctuations in laboratory temperature, humidity (Table 1) and participant hydration status, all factors that may alter microvascular blood flow and density. Attempts were made to limit these potential confounding factors by performing CytoCam-IDF imaging at the same time of day in heated purpose-built laboratories, and encouraging participants to maintain a good state of hydration. Previous studies at altitude have also raised concerns over the development of tissue oedema10,11 that can occur on ascent to altitude27. Whilst this could potentially reduce image quality and lead to false measurements of flow and density, our IDF camera provided us with a depth of focus reading, thus allowing us to confirm that we were recording at the same depth under the tongue on each time point. Finally, we have discussed alterations in capillary or vessel density. It is important however to clarify this nomenclature. IDF imaging cannot image blood vessels directly but rather uses the fact that polarized green light is optimally absorbed by red blood cells within the microvasculature regardless of oxygenation status. Absorption of light by haemoglobin, but not by surrounding tissues, therefore creates a distinct contrast of dark and light colour respectively, and red blood cells moving through the mucosal microcirculation thus appear as dark globules moving along the axis of flow. All vessels visualized are therefore only seen if they contain erythrocytes. The variables measured by IDF imaging (and its precursor SDF imaging) include a measure of total vessel density (TVD) and perfused vessel density (PVD)28. A distinction is made between the two depending on the speed of red blood cell flow within the observed vessels. TVD includes vessels which contain erythrocytes flowing at any velocity (or even at standstill), whilst PVD only includes vessels with continuously moving erythrocytes. As we cannot measure erythrocyte velocity with this simplified method, our observations therefore describe the TVD.
This study demonstrated an increase in sublingual microvascular vessel density on early and sustained exposure to hypobaric hypoxia; and, for the first time, that no significant change in vessel density occurred on immediate descent. The technique used to capture the images provided a rapid and reliable means for assessing changes in vessel density, and could be applied in future studies of microcirculatory vessel density. Further research in this area may allow a more complete comprehension of the multidimensional response to sustained hypoxia that occurs during pathophysiological situations.
F1000Research: Dataset 1. IDF values, 10.5256/f1000research.7649.d13402129
F1000Research: Dataset 2. Physiological values, 10.5256/f1000research.7649.d13402230
E G-K: design of study, collection of data, analysis of data, writing manuscript
JC: collection of data, analysis of data, writing manuscript
PH: analysis of data, writing manuscript
MG: design of study, writing manuscript
CI: design of study, writing manuscript
DM: design of study, analysis of data, writing manuscript
All authors have seen and agreed to the final content of the manuscript
Braedius Medical, a company owned by a relative of Can Ince, has developed and designed a hand held microscope called CytoCam-IDF imaging. Can Ince has no financial relation with Braedius Medical of any sort; he never owned shares, or received consultancy or speaker fees from Braedius Medical.
Xtreme Everest 2 was supported by the Royal Free Hospital NHS Trust Charity, the Special Trustees of University College London Hospital NHS Foundation Trust, the Southampton University Hospital Charity, the UCL Institute of Sports Exercise and Health, The London Clinic, University College London, University of Southampton, Duke University Medical School, the United Kingdom Intensive Care Society, the National Institute of Academic Anaesthesia, the Rhinology and Laryngology Research Fund, The Physiological Society, Smiths Medical, Deltex Medical, Atlantic Customer Solutions and the Xtreme Everest 2 volunteer participants who trekked to Everest Base Camp.
Some of this work was undertaken at University College London Hospital-University College London Biomedical Research Centre, which received a proportion of funding from the United Kingdom Department of Health’s National Institute for Health Research Biomedical Research Centers funding scheme. Some of this work was undertaken at University Hospital Southampton-University of Southampton Respiratory Biomedical Research Unit, which received a proportion of funding from the United Kingdom Department of Health’s National Institute for Health Research Biomedical Research Units funding scheme.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Xtreme Everest 2 is a research project coordinated by the Caudwell Xtreme Everest Hypoxia Research Consortium, collaboration between the UCL Centre for Altitude, Space, and Extreme Environment Medicine, the Centre for Human Integrative Physiology at the University of Southampton and the Duke University Medical Centre. Membership, roles and responsibilities of the Xtreme Everest 2 Research Group can be found at www.xtreme-everest.co.uk/team.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 30 Aug 16 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
The authors state that the CytoCam operates by emitting polarized light, this is incorrect.
... Continue reading Some important elements regarding design and textual accuracy were completely missed by the authors and the reviewers.
The authors state that the CytoCam operates by emitting polarized light, this is incorrect.
Assessment of capillary density has been validated before and there are numerous reports available in humans and animals that show the applications of capillary density measurements (e.g. by Lindeboom et al. 2005 and onwards). The type of data presented in the manuscript may be infrequently used for investigating the topics of the current report on hypobaric hypoxia, but not in other fields and was drawn entirely from oral medicine and dentistry. The authors unfortunately did not refer to the source publications correctly referencing reproducibility and validation of capillary density measurements. The two citations that were used do not report validations of the analysis methodology. Why was intra-class correlation data not presented in the Results section of the report?
The microcirculation data was not divided by 1.79 to present a standardized unit of capillaries per mm2, which is identified in several papers as cpll/mm2 for capillary density (CD) or functional capillary density (FCD), a completely different unit of analysis. As the data stands, it is not clear what the units are and it appears that the density data was interpreted as a number per 1.79 mm2 and not per mm2. Others might argue, but the only reason that dividing by 1.79 for capillary density counts is permissible for labial mucosa capillary density is because of counting of repeating (identical) vascular loops, a population count essentially, dividing by 1.79 would not work for branching vascular networks as in TVD or PVD.
Standardizing the data serves also another interesting role because the data from the CytoCam can then also be used to compare all previously obtained data with SDF imaging that also presents data on capillary density per mm2 in the visual field, this yields progress of the science and not always starting anew. Was normalization of the data thought of? One cannot assume that everybody has the same number of capillaries in their lips, some have more and others have less, that is why standardizing the data would allow clustered analysis and ease in trend assessments, especially when a repeated measurement in executed to trace the effects of a condition, in this case hypobaric hypoxia from ascending or return to normobaric normoxia from descent back to sea level.
The observations do not describe the consensus reports regarding TVD nor PVD, as detailed in the manuscript. TVD is reserved for network type (e.g. sublingual) analysis. This is why there should be different nomenclature for different anatomic compartments. Was there angioarchitecture classification performed, as presented by Weber et al. 2015? This classification provides a way of sorting the angioarchitecture and matching it with analysis methodology. For example, an array of capillary loops is a Class 1 angioarchitecture and fits with a field of view counting type analysis, a Class 2 is a hybrid between capillary loops and network vasculature and fits either a counting or AVA type analysis depending on the research question, and finally a Class 3 is exclusively network microcirculation fitting AVA and CCTools type analysis with TVD as mm/mm2. The data should be in total capillary density (TCD) or actually functional capillary density (FCD). The data of capillary density is not the same as TVD. Depending on the scientific or clinical questions the investigators are trying to answer, the design of the study should take into consideration whether the study objectives are to investigate mucosal state implicitly or translate measurements of the reticular layer of the oral mucosa as a model representing central or systemic microhemodynamics.
In the first line of the Discussion, the first line in the Conclusions and in two more instances in between the Discussion and Conclusions, the authors refer to measurements of sublingual microcirculation obtained in their report. The report describes lip (labial) mucosa microvascular density measurements and not sublingual microvascular measurements.
There are more publications that report on validation of the CytoCam instrument before the report by Hutchings S et al. 2015. Other topics that were mentioned such as neovascularization was not properly addressed.
Finally, to understand the oral measurements, some knowledge of oral physiology is recommended. For example, since the oral mucosa of both the upper and lower lips are peppered with minor salivary ducts, their salivary secretions and associated microcirculation are controlled by shifts in sympathetic and parasympathetic mechanisms that may not lineup with identifying changes in capillary density in for example dry mouth from breathing, cold temperatures, high altitude hypoxia and compensatory autonomic activity in response to low atmospheric pressures and/or hypoxia.
Addressing these points would strengthen the scientific integrity of this report and improve research design for future studies.
The authors state that the CytoCam operates by emitting polarized light, this is incorrect.
Assessment of capillary density has been validated before and there are numerous reports available in humans and animals that show the applications of capillary density measurements (e.g. by Lindeboom et al. 2005 and onwards). The type of data presented in the manuscript may be infrequently used for investigating the topics of the current report on hypobaric hypoxia, but not in other fields and was drawn entirely from oral medicine and dentistry. The authors unfortunately did not refer to the source publications correctly referencing reproducibility and validation of capillary density measurements. The two citations that were used do not report validations of the analysis methodology. Why was intra-class correlation data not presented in the Results section of the report?
The microcirculation data was not divided by 1.79 to present a standardized unit of capillaries per mm2, which is identified in several papers as cpll/mm2 for capillary density (CD) or functional capillary density (FCD), a completely different unit of analysis. As the data stands, it is not clear what the units are and it appears that the density data was interpreted as a number per 1.79 mm2 and not per mm2. Others might argue, but the only reason that dividing by 1.79 for capillary density counts is permissible for labial mucosa capillary density is because of counting of repeating (identical) vascular loops, a population count essentially, dividing by 1.79 would not work for branching vascular networks as in TVD or PVD.
Standardizing the data serves also another interesting role because the data from the CytoCam can then also be used to compare all previously obtained data with SDF imaging that also presents data on capillary density per mm2 in the visual field, this yields progress of the science and not always starting anew. Was normalization of the data thought of? One cannot assume that everybody has the same number of capillaries in their lips, some have more and others have less, that is why standardizing the data would allow clustered analysis and ease in trend assessments, especially when a repeated measurement in executed to trace the effects of a condition, in this case hypobaric hypoxia from ascending or return to normobaric normoxia from descent back to sea level.
The observations do not describe the consensus reports regarding TVD nor PVD, as detailed in the manuscript. TVD is reserved for network type (e.g. sublingual) analysis. This is why there should be different nomenclature for different anatomic compartments. Was there angioarchitecture classification performed, as presented by Weber et al. 2015? This classification provides a way of sorting the angioarchitecture and matching it with analysis methodology. For example, an array of capillary loops is a Class 1 angioarchitecture and fits with a field of view counting type analysis, a Class 2 is a hybrid between capillary loops and network vasculature and fits either a counting or AVA type analysis depending on the research question, and finally a Class 3 is exclusively network microcirculation fitting AVA and CCTools type analysis with TVD as mm/mm2. The data should be in total capillary density (TCD) or actually functional capillary density (FCD). The data of capillary density is not the same as TVD. Depending on the scientific or clinical questions the investigators are trying to answer, the design of the study should take into consideration whether the study objectives are to investigate mucosal state implicitly or translate measurements of the reticular layer of the oral mucosa as a model representing central or systemic microhemodynamics.
In the first line of the Discussion, the first line in the Conclusions and in two more instances in between the Discussion and Conclusions, the authors refer to measurements of sublingual microcirculation obtained in their report. The report describes lip (labial) mucosa microvascular density measurements and not sublingual microvascular measurements.
There are more publications that report on validation of the CytoCam instrument before the report by Hutchings S et al. 2015. Other topics that were mentioned such as neovascularization was not properly addressed.
Finally, to understand the oral measurements, some knowledge of oral physiology is recommended. For example, since the oral mucosa of both the upper and lower lips are peppered with minor salivary ducts, their salivary secretions and associated microcirculation are controlled by shifts in sympathetic and parasympathetic mechanisms that may not lineup with identifying changes in capillary density in for example dry mouth from breathing, cold temperatures, high altitude hypoxia and compensatory autonomic activity in response to low atmospheric pressures and/or hypoxia.
Addressing these points would strengthen the scientific integrity of this report and improve research design for future studies.