Keywords
accelerometer, biomarker, clinical assessment, diagnostic, gait, measurement
accelerometer, biomarker, clinical assessment, diagnostic, gait, measurement
Cohort/pathological studies need objective methods of capturing outcomes sensitive to disease onset and progression.
Gait has been shown as a pragmatic and useful (bio) marker of incipient pathology, inform diagnostic, track disease progression and measure the efficacy of interventions.
Wearable technology offers the ability to capture gait data in any environment.
A validated conceptual model of gait is presented. We recommend its adoption and use of a single low-cost wearable on the lower back with supplied analytical methodology.
Quantified gait characteristics with wearables facilitate the possibility for personalised treatment and integration into modern telehealth infrastructures.
Human locomotion (gait) can be described as the ability to perform a whole body movement in a rhythmical and consistent manner to transverse a distance in a safe and upright posture. Its preservation is important for independence and longevity in older adults and crucial for people with movement disorders whose quality of life is further threatened by falls and multisystem deconditioning1. Its correct quantification is now recognised as a powerful tool to identify ageing2, enhance diagnostics, measure efficacy of intervention and monitor disease progression2–4. Furthermore, its utility can be broadened to predict the risk of disease, falls, and cognitive decline5.
While gait speed is a useful global characteristic of performance6 it may not capture the nature of underlying pathology7. Instrumenting gait to define more precise and clinical relevant spatio-temporal gait features (e.g. step time, step length) stem from the use of large, expensive mechanical laboratory-based equipment typical of clinical/laboratory facilities. A newer more practical approach has emerged in the form of wearable technology (wearables), i.e. lightweight, discrete and smaller accelerometer and/or gyroscope-based devices that can be attached to the body over/under clothing. The added benefit of these devices is their suitability for deployment in any setting: low-cost, continuous recording for a multitudinous number of gait cycles8 and potential for quantifying novel frequency-based gait features9. Despite their obvious advantages, their use has been limited to academic studies rather than regular clinical usage within epidemiological studies. This can be attributed to: (i) poor agreement when compared to traditional laboratory-based reference equipment during validation studies8,10; and (ii) bespoke technical/engineering skills required to design/implement algorithms for the interpretation of the raw signals which differ due to attachment location, e.g. chest or waist11. The latter presents a signal processing challenge beyond the scope of any (typical) clinical researcher for whom the application of wearables would yield greater dividends: gait assessment as an accurate and reliable prognostic tool for healthy and/or pathological populations2,12.
In this tutorial we address this problem which has hindered both engineering and clinical professions: development versus application. We provide an introduction on how gait can be instrumented with a single, low-cost wearable. This is informed by best practice, validated methodologies8,10 and a clinically relevant conceptual gait model7. We hope this tutorial will facilitate the utility of instrumented gait as a pragmatic tool for biomarker development in future epidemiological studies.
The common sensor within modern wearables comprises a tri-axial (medio-lateral, anterior-posterior, longitudinal) accelerometer: due to low manufacturing cost, miniaturised size and low power consumption8. Data digitisation and associated memory within the wearable, one full battery charge of a modern wearable is sufficient to gather data every 0.01s (100 Hertz) for 7 days. The equivalent of over 180 million (60 data point/second × 3 axis) data points to analyse a participant. Accelerometers quantify acceleration (measured in meters per second squared, m∙s-2), calculated from the varying voltage generated within the sensor during movement (e.g. gait), for detailed functionality refer to 13. The signal generated is a combination of acceleration due to (i) dynamic conditions where each axis is perturbed due to 3-dimensional motion and (ii) static conditions where gravity has a pronounced effect on one axis of the tri-axial accelerometer (depending on attachment orientation) making this sensor useful for measuring static posture (lying, sitting, standing).
There is a plethora of commercial wearables for gait studies, e.g.: GaitUp (foot), Opal (ankle), StepWatch™ (shank) and DynaPort (lower back). Each of the aforementioned may not offer the high sampling rates to gather ~180 million data points but all positives/negatives depending on the research question and provision of pre-programed outcomes. Nevertheless, all may be constraint by proprietary software and hence inbuilt data analytics. However, a recent shift by manufacturers has seen the (intellectual property) shackles loosened/removed to allow access to the ‘raw’ wearable data for bespoke analysis, facilitating attachment to any anatomical location (e.g. Shimmer™)14,15. This has been driven by the rapidly developing ‘open-source movement’, a concept of allowing access to all technical schematics, software scripts and algorithm descriptions. As such the potential for researchers (engineering/clinical) to analyse and interpret wearable signals has risen. One open-source wearable is the movement monitor AX3 (from Axivity; dimensions: 23.0 × 32.5 × 7.6 mm; weight: 9 grams), which allows access to raw data and is not constrained by one anatomical location. While that device is low-cost, no proprietary software exists to aid analytics from the signals that are generated.
The following section details the instrumentation of gait in any environment. While numerous devices have been highlighted, we present a methodology for a high resolution device (100Hz) worn on the lower back.
Due to the miniaturised form factor of most wearables, they can be worn discreetly on almost any body location. As different accelerations are experienced at different anatomical locations, correct placement is of paramount importance when attaching the wearable11. This is because algorithms used to investigate the signal and compute spatio-temporal outcomes are dependent on signal characteristics such as repeatable signal shapes/features. Typically, gait research has aligned to use of wearables located as close as possible to the centre of mass (CoM), i.e. the lower back (typically, 5th lumbar vertebrae, L5). This best tracks whole body movement and for the purposes of instrumented testing a number of physical capability assessments and associated algorithms16. In another, it facilitates the use of a single wearable which reduces burden on the researcher and participant. This is of paramount importance during intervention or epidemiological studies where large patient numbers are recruited and tested12,17,18. The following details a methodology for instrumented gait analysis that has been successfully implemented in several healthy and pathological studies8,10,12,18–20.
Device attachment. Commercial devices are usually equipped with a strap/belt/clip for attachment. For the purposes of instrumented gait it is preferable that the wearable is attached as firmly to the participant as possible, eliminating spurious movement due to slippage. This usually requires direct attachment to the skin with a combination of dermatological adhesive(s) (e.g. Hypafix, BSN Medical Limited, Hull, UK) and double-sided tape. However, during prolonged testing, the participant’s skin (if frail/dry) can become compromised as a result of slight wearable movement due to lack of protection from thin double-sided tape. A solution is to adopt an adhesive hydrogel (e.g. PALstickies, PALTechnologies, Glasgow, UK) which provides additional padding due to its thicker design. Some motion artefact (slippage) and misalignment due to correct orientation and placement may be eliminated at the pre-processing stage from previously recommended procedures21,22. Generally, under controlled gait assessment motion artefact is minimised due to a stringent and structured protocol. (Note: alternate locations (e.g. chest, waist) may be possible, depending on the robustness (suitability) of the algorithm used to accurately detect gait events for different locations other than from its intended use20).
Protocol & gait characteristics. Validated instrumentation has shown that the use of a single wearable on L5 can capture 14 clinically relevant gait characteristics10,16. Derived from a conceptual model (Figure 1a) they have been shown to be sensitive to age and pathology2. Previous research suggests that the participant should perform a 2 minute continuous walk over a straight, or alternatively, looped path (Figure 1b) to record a sufficient number of gait cycles during steady state walking which improves the reliability of gait variability and asymmetry1,3. If steady state walking is required then the first 2.5 m of walking should be excluded23. If a testing environment doesn’t permit the use of a continuous walk, repeated intermittent walks and pooling of data is recommended. However, gait initiation/termination and their associated acceleration and deceleration periods may negatively influence results. This can be minimised by excluding the first and last steps (values) of the walks before pooling.

(a) A conceptual model of gait showing 5 domains and 16 characteristics, M, A and V refer to mean, asymmetry and variability, respectively. 14/16 characteristics can be replicated with a single wearable worn on L5, step width (mean and variability) cannot. (b) A suitable path to test gait. The (suggested) 25m loop shown has sufficient linear paths to sustain steady state walking, while the curvilinear paths should be shallow enough to avoid abrupt directional changes.
Data import & segmentation. Matlab® is a scripting programming language for general scientific computing that utilizes matrix oriented high-level programming for a large number of numerical tasks on many common platforms. Data processing can be achieved using existing and/or prototypic algorithms via script or command structure interfaces24,25. Its support network (‘Matlab Central’), comprehensive toolboxes and ability to be translated to open-source languages (e.g. PythonTM, Octave) make it suitable for the processing of (gait) data into other programming software types26–29. Therefore, for the purposes of this tutorial Matlab® pseudo-code is provided.
Data must be downloaded from the wearable via associated software and saved securely. Data recorded by the wearable and saved by the proprietary software (including open-source) will typically be made available as a comma separated value (.csv) file due to its exchangeability. Importing the data to Matlab® (Appendix 1, 1) can be achieved through the use of the xlsread function which offers the freedom to import data from a single or multiple column array(s) within a specified spreadsheet (Appendix 1, 2).
Once imported, data will automatically be saved to Matlab® workspace as a variable. Typically some generic movement data will be recorded by the wearable during a testing session before/after the gait task and will need to be removed. If saved via a spreadsheet, erroneous data can be highlighted and deleted, trimming the data. If intermittent walks were performed, data can be segmented manually in the spreadsheet format prior to importing. (Note: Those familiar with Matlab®, the ginput function can be used to segment data; enables user to define the exact start/end of the walk due to cursor point and click on a plot and save the x-axis values (samples/frames), Appendix 1, 3).
Data preparation: pre-processing. Data captured by wearables are subject to ‘noise’: random fluctuations in the signal due to connecting hardware and/or external interference. Removing noise can be achieved by filtering. There are many techniques one can apply to a signal (e.g. Butterworth, Chebyshev), each with their own advantages/disadvantages. Essentially, filters are deemed useful depending on how well they can remove the unwanted signal due to various associated parameters. Care must be taken when choosing those values as it may impact algorithm analysis, feature extraction. Nevertheless, the literature details the most common method as the 4th order Butterworth filter with a cut off-frequency between 15–20 Hertz (Hz), Appendix 1(4). (For a comprehensive assessment of pre-processing of wearable gait signals refer to 30).
Correcting for offset & misalignment. When the wearable is attached to the participant, it is generally understood that the orientation or alignment of the device is offset due to attachment error and participant body shape. Additionally, gravity exerts a force, most notable on one axis. Attachment error and gravity can be easily overcome by asking the participant to remain still upon initial attachment and recording a few seconds of (quasi) static activity in a standing posture. The average/mean of the values captured by each axis in this posture is later subtracted from corresponding axes to eliminate offsets and misalignment.
However, this method is best suited to correct acceleration data in static postures only and not recommended for post-processing of gait data22. The correct approach is to transform the tri-axial data into a horizontal-vertical orthogonal coordinate system, i.e. using trigonometry relating to the Cartesian coordinate system22,30. The methodology relies on calculating and correcting for the best estimates of the (offset/misalignment) angles (θ) between the true horizontal-vertical and that of the raw anterior-posterior (aa) and medio-lateral (am) accelerations. While the accelerometer within the wearable cannot provide the rotational angle (gyroscopes), it is deduced22 that the average value of aa and am will approach the sin of the angles within the same directions, Equation 1–Equation 4 (translated code Appendix 1, 5). By applying the inverse sin (arcsin) methodology, one can derive the necessary values needed to correct offset/misalignment in four straightforward, recommended30 steps:
(i) Correction in the anterior-posterior plane (aA, note change of subscript case):
aA = aa cos θa – aν sin θa (1)
(ii) An interim correction (a’ν) in the vertical direction must be derived before a true value for aV:
a′ν = aa sin θa + θν cos θa (2)
(iii) Interim values in the vertical direction used to derive aM
aM = am cos θm – a′ν sin θm (3)
(iv) Finally, aV may now be estimated:
aV = am sin θm + a′ν cos θm – 1g (4)
The above is achieved through mean, sin, cos and arcsin functions along with basic matrix multiplication (Appendix 1, 5).
Algorithms. Methodologies have been developed to quantify temporal and spatial characteristics for a wearable on L5, comparisons can be found here31. All aim to identify two features of gait: initial contact (IC, i.e. heel strike) and final contact (FC, i.e. toe off), Figure 2a. A robust temporal method31 uses wavelets32. This methodology is a powerful signal processing tool that has been used successfully in gait and postural transition analysis32–34, yet its use remains limited due to complexity. The basic premise is that it offers an extension on the Fourier transform by two procedures: continuous (CWT) and discrete (DWT) wavelet transforms. Detailed descriptions is beyond the scope of this manuscript, but can be easily described; (i) CWT: a correlation between waveforms (raw signal and probing function, i.e. wavelet) at different scales (~ frequencies) and positions (in time), where the resulting coefficients roughly correspond to the best match; and (ii) DWT: a combination of high/low pass filters to divide up a (raw) signal into various components. (see 35 in depth descriptions refer to). Nevertheless, implementing a CWT algorithm32 for IC/FC event detection can be relatively straightforward if utilising the Wavelet Toolbox within Matlab®, Appendix 1(6):
(i) Numerical integration of the raw vertical acceleration (av) with the function cumtrapz
(ii) Differentiation of the integrated signal with the cwt function (Wavelet ToolboxTM Matlab®) resulting in signal S1, Figure 2b
(iii) Find S1 local minima times, which equate to IC, through the use of the findpeaks function, Figure 2b
(iv) Differentiate signal S1 with cwt function to get signal S2,
(v) Find local maxima (FC) times of signal S2 by using findpeaks, Figure 2b
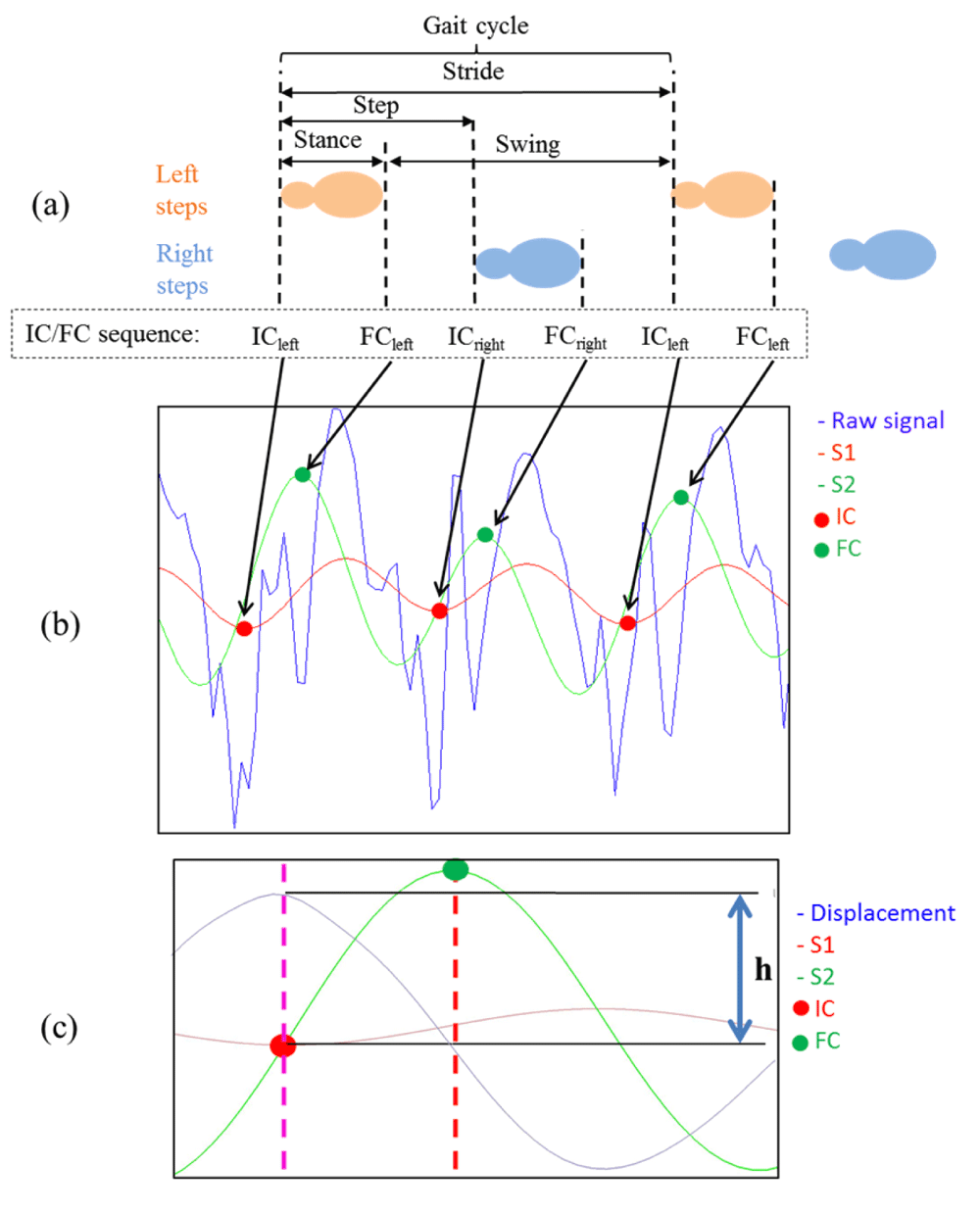
Gait signal from a young healthy adult (a) The gait cycle with depictions of stride, step, stance and swing characteristics from the IC/FC events (b) The raw signal (av), integrated and differentiated CWT signals with corresponding IC/FC events. The IC/FC sequence must be amalgamated into one numerical array from the alternating peaks/troughs to estimate the correct timing sequence for stride, step, stance and swing times. (c) Step length can be derived using Equation 5, where h is derived from change of wearable height due to double integration of vertical acceleration (implementing cumtrapz function twice).
Temporal characteristics. To fully replicate the characteristics of gait: step, stance, stride and swing times must be derived. This is achieved through the sequence of IC/FC events in relation to the double support phase of the gait cycle (see Figure 2). From the sequence (i) of IC/FC events, both left and right (opposite) events are identified, and subsequently step, stride, stance and swing times are estimated (Equation 5–Equation 8). For full details of calculating these parameters see 10,36.
Spatial characteristics. A spatial algorithm based on the inverted pendulum model tracks the CoM37. However, the model is reliant on a known variable, wearable-height. This manual component is a weakness: requiring a known input and can have weak accuracy for step length or total distance walked8,12. Yet it remains a useful metric to compute via the simple relationship shown in Equation 5, where l is wearable height and h is change in height of the wearable (i.e. CoM) as the participant walks, Appendix 1(7). Subsequently, by fusing the algorithms from Figure 28,10, it is possible to quantify an estimate for step velocity (Equation 6 and Appendix 1, 7). However, implementing the cumtrapz function to derive velocity and speed from acceleration introduces an error known as drift. This can be eliminated through the use of filtering, but generally remains problematic within wearable gait analysis.
Variability and asymmetry characteristics. It is useful to distinguish between left/right step characteristics for variability and asymmetry outcomes (Equation 11 a, b and 12, Appendix 1, 8) in asymmetrical diseases38. Differentiating between left/right during a long continuous walk is easier (assume first as left or right and alternate values thereafter) compared to repeated intermittent walks when (for robustness) it would be recommended to note what foot was used for initiation8. Alternatively, a protocol could request the participant initiates walking with the same foot. Subsequent assignment of values to left/right can be made during data analysis by manually dividing the data. (For the readers interest, left/right steps may be identified by automated but more complex algorithms and can be found here: 32,37). Correct calculation of variability1,10 and asymmetry is performed by:
or
Variability= SD(Steps) (7b)
Asymmetryleft & right = |averageleft – averageright| (8)
Our aim in this paper has been to present an introductory tutorial, learned from best practice and robust methodologies to instrumented gait with a single wearable. Drawing on a validated conceptual model we provide a suitable and robust means to quantify and implement an analysis framework to derive 14 clinically relevant gait characteristics, for quantification in any environment. This has practical implications for the understanding of instrumented gait in future epidemiological studies, as a useful diagnostic.
It is important to consider the limitations associated with a single tri-axial accelerometer wearable. Direct integration of the raw acceleration data can amplify errors in calculation and compromise the integrity of results. Raw acceleration data varies among controls and across pathologies, as such universal processing (algorithms) recommendations are difficult to derive39. Location of the wearable in this example is specific to the algorithms’ functionality and therefore gait outcomes quantified from alternation locations should treated with caution20.
Though implementing the algorithm and associated signal processing techniques can seem straightforward, initial familiarisation with the scripting language(s) and implementation of code can be daunting. Nonetheless, the methodologies presented here provide an opportunity to add more informed, objective data to future epidemiological studies. Wearables are being increasingly used in free-living environments, richer in habitual behaviours and aligning with developing telehealth infrastructures5,12. Understanding the abilities as well as the limitations of existing technologies by all professions can help harmonise technological resources and find application in alternate fields of research.
F1000Research: Dataset 1. Raw data for 'Instrumented gait assessment with a single wearable', 10.5256/f1000research.9591.d13536941.
SDD, AH, SS and AG conceived the methodology and drafted the paper with help from CL, PE and AB. LR developed the protocol and advised on best clinical practice with SS. SDD, AH and AG performed the scripted Matlab® algorithms with input from PE and AG. All authors contributed to critical revisions of the manuscript including methodology for the purposes of an introductory tutorial into instrumented gait.
SDD, AG and LR are supported by the Biomedical Research Unit/Centre at Newcastle University. The research was also supported by the NIHR Newcastle CRF Infrastructure funding. AB is supported by the ADAPT, a Norwegian Research Council funded project (FRIMEDBIO, 230435). Views expressed are solely those of the authors.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Listing | Code and descriptions |
|---|---|
| 1 | General scripting The different font text (below) may be input to the Command Window of Matlab®. Wording within Matlab® (like most scripting languages) is case-sensitive e.g. “Variable” ≠ “variable”. Words appearing after the ‘%’ are ignored, this is a useful method to insert comments within code for clarity. It is important to remember that Matlab® deals with rows and columns of data. For example the variable Data, a 100 (row) × 4 (column) matrix, Column 1 = Data(:,1); Row 57 = Data(57,:); Row 12, column 3 = Data(12,3); For further information refer to the ‘Matlab Central’ file exchange. It is a useful resource with many tips and code not already detailed within Matlab®. Within the package is a useful ‘help’ functionality that will inform about any code i.e. mean, standard deviation, integration. Moreover, it will (usually) detail how the named function is best applied with examples. When creating variables, its best to use descriptive names such as outcomes, e.g. ‘ParticipantHeight’ (no spaces). The variable name is stored/displayed in the Workspace. Typically data are stored as a numerical array (rows, columns) and are manipulated through by the functions within the package. ‘help mean’ returns detailed usage of the mean function and how the mean of an array of values may be found. Within the help text will be suggested functions similar to what was queried, in this instance median, std*, min, max, var*, cov*, mode are displayed. (*standard deviation, variance and covariance) |
| 2 | Importing (reading) data AccData = xlsread(‘GaitData.xls’, ‘Sheet1’, ‘A1:C1000’); The variable created (AccData, accelerometer data) is to the left of the equation. Data are written to that variable from a file (‘GaitData.xls’) within the sheet (‘Sheet1’), 3 columns wide (spreadsheet columns A to C; tri-axial data) and 1000 lines long, i.e. gait sampled at 100 Hz (0.01 times a second) for 100s. |
| 3 | Plotting and manual segmentation Use of the ginput function will accompany plot where the gait data are displayed via a figure. Plot allows the researcher to visualise the data, ginput to specifically highlight the start and end of the walk. Suggested use of plot and with ginput: plot(AccData); % plots tri-axial accelerometer data legend(‘Vertical’,’Medio’,’Antero’); % attaches a legend to the plot figure/window xlabel(‘Samples’); ylabel(‘Gravity (g)’); % labels x and y StartStop = ginput(2); % saves 2 x and y coordinates from user input clicking on plot For the purposes of defining start/end points within accelerometer data, refer to the 1st column of StartStop,as follows: StartStop(:,1); |
| 4 | Filtering The 4th order Butterworth filter is the most common within the literature, with a cut off frequency of 15–20Hz (usually accepted to capture human movement. Filters induce lag within a signal, i.e. delay or distortion, which must be corrected by running back through the filter. [B,A] = butter(4, Wn); % 4th order, Wn is cut off freq., correct to half sampling rate Run forward/backward using filtfilt to avoid distortion: AccDataV = AccData(:,1); % Assign vertical acceleration, column 1 of AccData AccDataFilt = filtfilt(B, A, AccDataV); % Will generate the required filtered data |
| 5 | Acceleration correction to horizontal-vertical frame22 Aa = AccData(:,2); % Assign aa direction, column 2 am = AccData(:,3); % Assign am direction, column 3 avMean = mean(av); % Mean of av as best estimate of sin(θv) amMean = mean(am); % Mean of am best estimate of sin(θm) aA=aa*cos(asin(aaMean)) – av.*aaMean; % Equation 1 avv=aa*aaMean+ asin(aaMean).*cos(asin(avMean)); % Equation 2 aM=am*cos(asin(amMean)) – avv.*amMean; % Equation 3 aV=am*sin(asin(amMean)) + avv.*cos(asin(amMean))–1; % Equation 4 |
| 6 |
Integration and peak detection
av = AccData(:,1); % av assigned to column 1 Integratedav = cumtrapz(av); % Numerical integration of av fs = 100; % Define sample frequency as 100Hz CWTIntegratedav = cwt(Integratedav, 10, ‘gaus1’, 1/fs): % using Gaussian CWT at scale 10 The derivatives are calculated by a weighted average which corresponds to a smoothing function dependant on the scale and negative sign of the CWT40. [Peaks, Locations] = findpeaks(S1); % Locates the Peaks (values) and locations (time/samples) IC can now be assigned to Locations: IC = Locations; % Peaks may need examination, depending age, pathology10. |
| 7 | Calculating temporal gait characteristics from the IC/FC events Typically this is achieved by subtracting IC events from following IC/FC events within a looped array, undertaken by for/end loop as shown below. An array of SwingTime values can be estimated externally from the loop once all the stride and stance values have been calculated. for i = 1:length(IC) % The for loop must be iterate a predefined StepTime(i) = IC(i + 1) – IC(i); StanceTime(i) = FC(i + 1) – IC(i); StrideTime(i) = IC(i + 2) – IC(i); end SwingTime = StrideTime – StanceTime; |
| 8 | Integration and spatio-temporal estimations hvel = cumtrapz(av); % Integrate to estimate velocity, h = cumtrapz(hvel); % further integration will derive position StepLength = 2(sqrt(2*(Wearable Height) *h – h^2)); % Inverted pendulum, Equation 5 StepVelocity = Step Length/Step Time; % step velocity estimate, Equation 6 |
| 9 | Variability and asymmetry calculations Allocate left (first step within StepTime array) and right steps: StepTimeLeft = StepTime(1:2:end,:); % from 1st data point to end StepTimeRight = StepTime(2:2:end,:); % from 2nd data point to end Step time variability & asymmetry: StepTimeV = sqrt((var(StepTimeLeft)+var(StepTimeRight))/2); % Equation 7a or StepTimeV = std(StepTime); % Equation 7b and StepTimeA = abs(mean(StepTimeLeft)–mean(StepTimeRight)); % Equation 8 |
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
References
1. Jurman D, Jankovec M, Kamnik R, Topič M: Calibration and data fusion solution for the miniature attitude and heading reference system. Sensors and Actuators A: Physical. 2007; 138 (2): 411-420 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 14 Sep 16 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)