Keywords
Saccharomyces cerevisiae, centromeric plasmid, auxotrophic markers, self-establishing metabolically cooperating (SeMeCo) communities, metabolism
Saccharomyces cerevisiae, centromeric plasmid, auxotrophic markers, self-establishing metabolically cooperating (SeMeCo) communities, metabolism
Auxotrophic markers are single gene perturbations of essential metabolic pathways, that are exploited in the efficient selection of strains, plasmids and genome editing. Further, they are used in a diverse spectrum of genetic technologies, as their selection is efficient, their use economic, and in contrast to antibiotic selection markers, they do not revert by mutation1–3. In budding yeast, auxotrophic marker alleles important for histidine, leucine, uracil, methionine, lysine, adenine and tryptophan metabolism have been crossed or cloned into the popular S. cerevisiae laboratory strains. Harbouring 5 auxotrophic marker mutations, his3Δ1, leu2Δ0, ura3Δ0, lys2Δ0 or met17Δ04–9, strains derived from the S288c background served as the parents of the yeast gene-deletion collection5, and subsequent genetic libraries that are based on this principle. These libraries include gene deletion mutants5,10,11, genetically introduced GFP, GST, and TAP fusions12–14, transposon insertion mutants15, decreased abundance by mRNA perturbation (DAmP) mutants16, Tet-promoter controlled expression17 and the ts-alleles for essential genes18,19. Furthermore, systematic strain collections of other fungal species including Schizosaccharomyces pombe20,21 and the pathogens Candida glabrata22, Candida albicans23 or Neurospora crassa24, all involve use of auxotrophic markers as well. As a result, auxotrophic backgrounds are omnipresent in a large number of functional genomic experiments, and have been used in a countless number of small-scale experiments, resulting in their ubiquity across yeast molecular biology literature.
In order for a metabolic gene to function as an auxotrophic marker, it needs to be part of a metabolic pathway for which the cells possess an extracellular uptake and a sensing mechanism for the product of the interrupted pathway. Auxotrophic marker mutations are hence associated with metabolites that are readily taken up from the environment. This includes the metabolites exchanged between cells in communal growth, in particular amino acids25. The biosynthesis of amino acids accounts for up to half of the metabolic flux towards biomass, with amino acids making up to 2/3rds of the total mass of polar metabolites26,27. As a consequence, a shift from self-synthesis to uptake, as enforced by auxotrophy, is not without biological consequence. In fact, most of the genome-wide gene expression is sensitive to epistatic interaction within the Saccharomyces metabolic-genetic background28. The physiological effects arising from auxotrophy and complemented marker genes have been highlighted by several yeast labs for more than a decade2,29–33. Most importantly, to grow auxotrophic strains, amino acids and nucleotides need to be added to the growth medium. Nutrient supplementation affects not only the interrupted pathway itself, but the biosynthesis of other essential compounds, in particular the enzymatic cofactors, due to the metabolic network responding to perturbation at the systems levels, and hence, affecting multiple metabolic pathways in parallel2,34,35. Cell growth has consistently shown to be affected by nutrient supplementation, reflecting the variation in energy costs between biosynthesis and uptake/incorporation of the provided nutrients2,34. In batch cultures, supplements are also consumed at different rates33. As a consequence, nutrient availability changes during batch culture growth, rendering cells physiologically different between growth phases. In classic molecular biology, the use of a matched auxotrophic background as a wild-type control has been considered sufficient to account for the effects of auxotrophy2. Transcriptomic, proteomic and metabolomic analysis of complemented auxotrophs show however that this is not the case; the metabolic background deficiencies interact epistatically with the majority of the coding genome and in a context dependent manner. The biological explanation for this phenomenon is that metabolism is intrinsically intertwined with the gene expression machinery and is dependent on metabolic flux distribution. The same gene deletion introduced in a different auxotrophic background can hence cause an entirely different transcriptional response, so that a matched parent background is not able to compensate for these effects28.
We here present a series of single copy plasmids derived from the pHLUM minichromosome, which can be used for restoring prototrophy as well as for testing the metabolic capacity of budding yeast, by compensating for the possible combinations of his3, leu2, ura3, met17 (or lys2) deficiencies. For their use in S. cerevisiae, the plasmids contain a centromeric origin for single copy expression and express the marker genes under native S. cerevisiae promoter sequences39. To exploit multiple markers to reduce the plasmid segregation problem for protein expression experiments, we further introduced the uniform multiple cloning site of the pRS300 vector series. For cloning and manipulation in E. coli, the shuttle vectors contain a bacterial high-copy replication origin (pUC) and an ampicillin antibiotic resistance marker. Finally, the pHLUM series of the plasmid contains an N-terminal fragment (α-peptide) of the E. coli beta galactosidase (lacZ), for blue-white selection in appropriate cloning strains40, and an F1 origin for use in phage libraries. These plasmids can be used for complementing unused auxotrophies in laboratory yeast stains, to express proteins exploiting multiple parallel selection markers, and to study metabolite exchange interactions in synthetic yeast communities.
Escherichia coli strain DH5α was used as plasmid host and strains containing the recombinant plasmids were selected on LB medium with ampicillin (100 µg/ml) and grown at 37°C. Two commonly used S. cerevisiae strains in the S288c background, BY4741 (MATa his3Δ1 leu2Δ0 met17Δ0 ura3Δ0) and BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0)4, were used to test for genetic complementation of auxotrophic requirements by the plasmids created. The strains were grown in YPD (2% Glucose, 20 g/l peptone (Bacto™), 10 g/l yeast extract (Bacto™)) or synthetic minimal (SM) medium (2% glucose, 6.8 g/l yeast nitrogen base), as indicated. To enable growth of auxotrophic strains, the SM medium was supplemented with 20 mg/l histidine, 60 mg/l leucine, 20 mg/l uracil, 20 mg/l methionine and/or 50 mg/l lysine as indicated.
For site directed mutagenesis the Quikchange Lightning kit (Agilent) was used according to manufacturer guidelines, taking 50 ng of plasmid DNA as a template and 6.3 µM of each oligonucleotide (primers O09-O12, Table 1) to a total volume of 25 µl. The manufacturer's recommended cycling parameters, with a 2.5 min extension time, were followed.
All other enzymes for molecular cloning were purchased from New England Biolabs (NEB) and used as instructed. Genomic DNA was extracted from yeast with repeated freeze-thawing of cells in a lysis buffer as per previous publication36. DNA from genomic and plasmid templates was amplified with Phusion High-Fidelity DNA Polymerase (Finnzymes) supplemented with the CES combinatorial enhancer solution to increase primer specificity as described previously37.
Plasmids were isolated both from E. coli and S. cerevisiae with the QIAprep Spin Miniprep Kit (Qiagen). For the latter, a Qiagen protocol (Michael Jones, Chugai Institute for Molecular Medicine, Ibaraki, Japan, https://www.qiagen.com/gb/resources/resourcedetail?id=5b59b6b3-f11d-4215-b3f7-995a95875fc0&lang=en) was used. The protocol employs 425–600 μm acid-washed glass beads (Sigma) for mechanical lysis (30 sec, 6.5 M/s in a FastPrep®-24 Instrument (MP Biomedicals)). For homologous recombination to construct pLUK, two complementary primers were designed with 5’ and 3’ leader sequences homologous to plasmid 5’ and 3’ of the HIS3 marker gene (primers O15-O16, Table 1). The oligonucleotides were annealed by heating to 95°C then gradual cooling to RT. The plasmid pHLUK was linearized by cutting inside the HIS3 sequence with MscI. The yeast strain BY4741 was transformed with 100 ng of cut vector and 500 ng of annealed primers and was selected on SM medium supplemented with histidine. Clones with successful homologous recombination events were identified by failure to grow on SM medium without histidine.
Yeast strains were transformed with a high efficiency lithium acetate, PEG, salmon sperm protocol using 300 ng of plasmid per reaction38.
For the genetic complementation of the commonly used auxotrophic lesions in HIS3, LEU2, URA3 and MET17 we have previously constructed the pHLUM minichromome (Addgene ID 40276)33. The plasmid is based on pRS31339, contains a centromer, and an autonomous replication sequence, and the HIS3 marker as derived from the pRS313 backbone. The additional marker genes LEU2, URA3 and MET17 were cloned from other popular yeast plasmids (pRS42539, p426GPD41 and pRS4114 and were placed between unique restriction sites, so that they can be individually excised33, primers O01 - O06, Table 1). The three-gene insert is flanked by BamHI and XhoI and unique restriction sites AscI and SphI were designed between LEU2/URA3 and URA3/MET17, respectively, to allow for selective excision of the individual markers. However, in this original version of pHLUM, the HIS3 marker cannot be removed in a straightforward manner.
In order to improve the usefulness of the minichromosome, we decided to redesign the plasmid backbone, replacing all 4 marker genes but leaving the multiple cloning site of pRS313 intact. With a site directed mutagenesis strategy, we added an AatII restriction site 5’ and XhoI and BamHI sites 3’ of HIS3 (primers O09-O10, Table 1). In the same reaction we eliminated the BamHI and XhoI recognition sites from the multiple cloning site by exchanging two bases and preserving the sequence of the lacZ ɑ-peptide (primers O11-O12, Table 1). With the new restriction sites available and the absence of XhoI and BamHI in the multiple cloning site, the DNA fragment containing LEU2, URA3 and MET17 could be excised from pHLUM with XhoI and BamHI and integrated 3’ of the HIS3 gene on the modified pRS313 vector. The resulting plasmid was named pHLUM (plasmid HIS3 LEU2 URA3 MET15) (version 2). It maintains 8 unique endonuclease recognition sites in the multiple cloning site and the capacity for colorimetric lacZ complementation assays (Figure 1A).
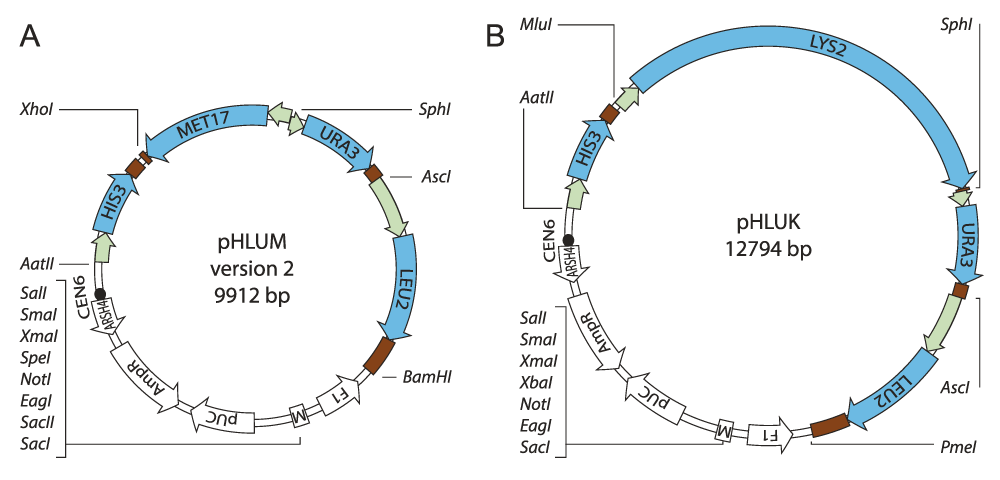
Physical maps of pHLUM (version 2) and pHLUK minichromosomes, the centromeric parents for the generated S. cerevisiae vector series. pHLUM (version 2) expresses HIS3, LEU2, URA3 and MET17 to complement auxotrophies in BY4741/MATa strains of the knock-out collection, while pHLUK expresses HIS3, LEU2, URA3 and LYS2, for the BY4742/MATα series.
In the typical MATα derivatives of the S. cerevisiae gene deletion collection (i.e. BY4742), LYS2 is deleted while the MET17 marker is wild-type. We used pHLUM (version 2) as a template and exchanged the MET17 marker with LYS2 to create an analoguos vector series (pHLUK). The LYS2 coding sequence contains recognition sites for both XhoI and BamHI. We therefore removed the BamHI site from pHLUM (version 2), and introduced at the same position a recognition sequence for PmeI. To this end we synthesised two complementary oligonucleotides (O13-O14, Table 1) and annealed them by heating to 95°C and gradual cooling to RT to yield a small double stranded DNA segment containing a PmeI site and cohesive ends to the BamHI digested pHLUM (version 2). The digested vector was dephosphorylated with Antarctic phosphatase (NEB), the annealed primers phosphorylated with polynucleotide kinase (NEB) and then ligated by T4 DNA ligase abolishing the recognition site for BamHI. Next, we amplified the LYS2 gene from BY4741 genomic DNA (O07-O08, Table 1) including the promoter and terminator regions according to the yeast promoter atlas42. Primer O07 contained recognition sites for SalI and MluI and primer O08 for SphI (Table 1). The modified plasmid was digested with XhoI and SphI and the MET17 marker replaced with the LYS2 PCR product digested with SalI/SphI. The cohesive ends of SalI and XhoI DNA fragments are compatible and abolish their recognition sites upon ligation. The MluI site allows digestion of the vector between LYS2 and HIS3 and excision of either marker (Figure 1B).
The unique endonuclease recognition sites between each of the marker genes facilitated the creation of the 21 derivatives of pHLUM (version 2) and pHLUK containing between 1 and 3 marker genes, in all possible combinations. The marker genes were excised by digestion with appropriate endonucleases, the 3' and 5’ overhangs were removed or filled in with T4 DNA polymerase and the plasmid ligated with T4 ligase (Table 2, Figure 2). The plasmid pLUK was generated using homologous recombination in yeast.
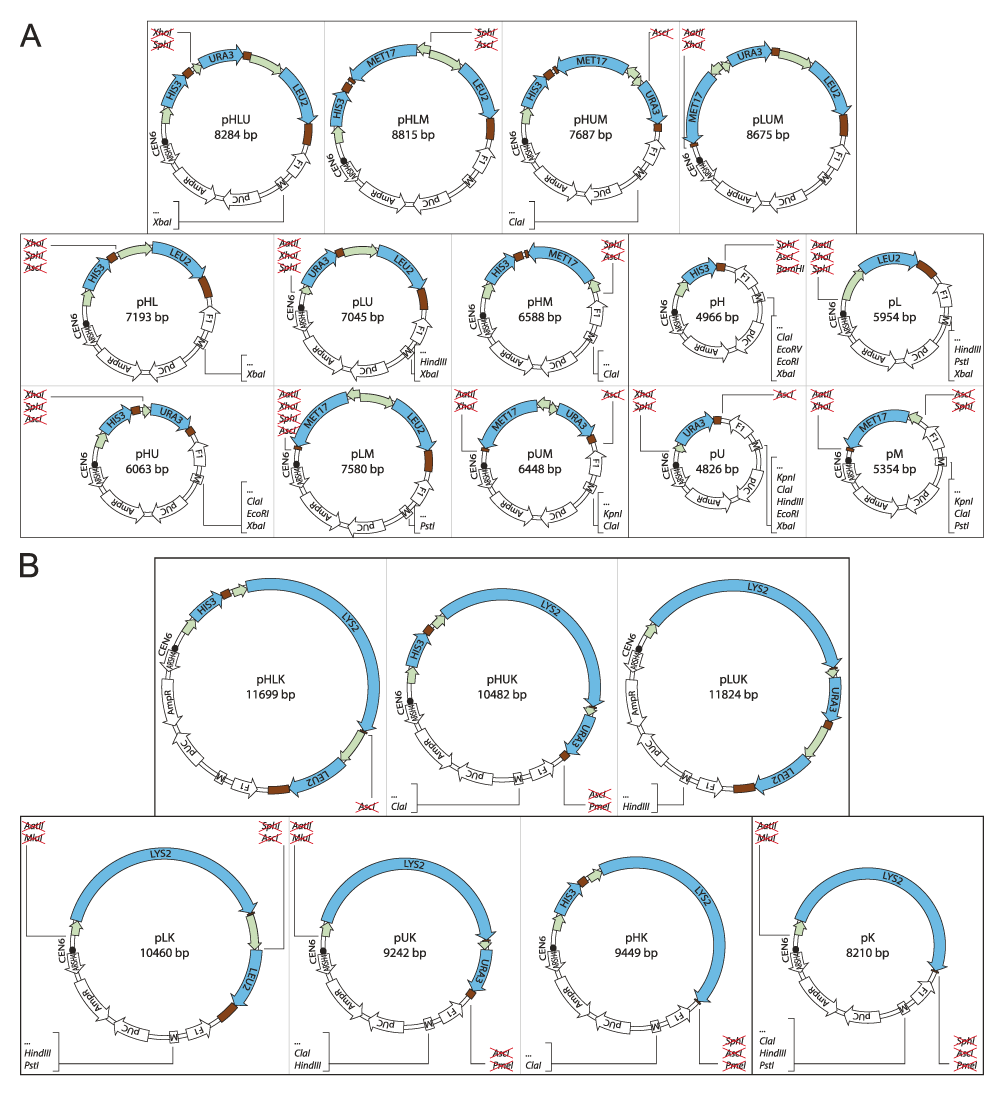
(A) The plasmids are generated from pHLUM (version 2) containing HIS3, LEU2, URA3 and MET17, and (B) pHLUK containing HIS3, LEU2, URA3 and LYS2 expressed under control of S. cerevisiae promoter and terminator sequences. Unique restriction sites between the marker genes and in the multiple cloning site (M) are indicated in the parent pHLUM (version 2) and pHLUK (Figure 1). Loss or acquisition of unique restriction sites is highlighted in the individual vector maps.
The completed plasmids were re-sequenced, which led to some corrections compared to the Genebank deposited version of pRS313 (GenBank: U03439.1) (Supplementary material). Successful genetic complementation of auxotrophic markers is illustrated upon transforming BY4741 and BY4742 strains, with the generated plasmids, and subsequent scoring of their growth on selective media for histidine, leucine, uracil and methionine or lysine, respectively. The plasmids restored all auxotrophies in BY4741 and BY4742 in the desired combination (Figure 3). Further, we tested the functionality of the lacZ α-peptide sequence retained in the pHLUM (version 2) series for blue-white selection, by transforming them in DH5α (Figure 3C). On X-Gal containing medium, a blue colour shift was observed.
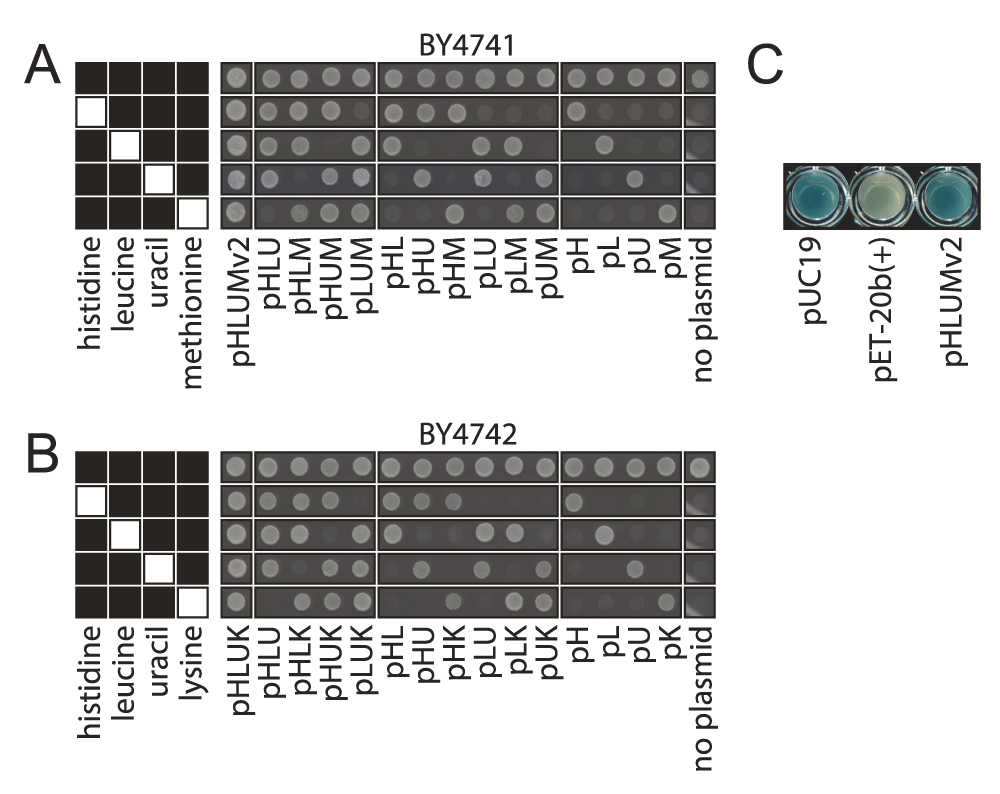
(A) S. cerevisiae haploid strains, BY4741 (MATa his3Δ1 leu2Δ0 met17Δ0 ura3Δ0) and (B) BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0)4 were transformed with each of the 23 centromeric plasmids from the pHLUM (version 2) and pHLUK series and spotted onto synthetic minimal medium containing four or all five supplements of 20 mg/l histidine, 60 mg/l leucine, 20 mg/l uracil, 20 mg/l methionine or 50 mg/l lysine, as indicated in black in left hand key. (C) Use of the plasmid series for colorimetric lacZ assays: DH5α transformed with the lacZ containing plasmid pUC19, the lacZ missing plasmid pET-20b(+) and pHLUM (version 2) (pHLUMv2), parent of 23 plasmid series. A shift from white to blue on X-Gal containing LB medium due to the presence of a partial lacZ sequence.
Due to the physiological impact of auxotrophy one would in an ideal world conduct all yeast experiments in prototrophic backgrounds and, if the objective of the experiment is to study a physiological process, use cells grown in minimal nutrient medium. However, most existing Saccharomyces lab strain resources are auxotrophic, and a majority of genetic techniques depend on the ability to select with genetic markers. The switch to antibiotic resistance markers is not a viable alternative to auxotrophies in many cases, as antibiotics can be expensive, are prone to persistence of sensitive cells, and by interfering with translation or transcription, have strong biological effects on their own43,44. We have noticed in our previous work, that a useful workaround, or compromise, for many applications is to complement the unused auxotrophic marker mutation with a multi-gene containing, single copy, centromeric single copy plasmid (minichromosome) that compensates for metabolic deficiencies present in the cell28,33. By nature, introducing an episome adds a new constraint due to its segregation. However, we found that the four metabolic genes on the pHLUM minichromosome provide selective advantage also under nutrient-rich growth conditions, so that cells retain the vector even in the absence of selection pressure33. Further, we tested for copy number effects, and found that the expression of HIS3, LEU2, MET15 and URA3 from the minichromosome fully suffices the biosynthetic needs33. A situation in which all cells are provided with a high concentration of nutrients, as would occur with media supplementation, may also be less native to cells community where, usually, a certain fraction of cells are dependent on metabolite exchange25,45. For the typical experiment, the constraints arising from segregation of a single-copy minichromosome that restores auxotrophy, are hence much smaller compared to the problems caused by the use of nutrient supplemented media and auxotrophic strain backgrounds.
To support the work with prototrophic yeasts, we present here 23 minichromosomal vectors for restoring prototrophy in popular laboratory strains of budding yeast. These plasmids compensate for histidine (HIS3), leucine (LEU2), uracil (URA3), methionine (MET17) and lysine (LYS2) deficiencies and combinations thereof, which have been introduced into many yeast strains derived from the S288c background. Furthermore, the multiple cloning array is compatible with the widely used pRS300 plasmid series and provides unique restriction sites to facilitate cloning of genes of interest. The different marker genes of these vectors also enable expression analysis in various genetic backgrounds. The main intended application of these plasmids is to restore auxotrophy in laboratory strains and to be able to conduct experiments in minimal nutrient supplemented medium. In this way, the effect of amino acid and nucleotide biosynthetic metabolism, which is responsible for a major fraction of the metabolic flux of a cell, as well as has a profound impact on gene expression and physiology28, can be studied.
Another application for these plasmids is to study metabolic cooperation in self establishing yeast communities (SeMeCo). It has been known for a long time that a subpopulation of plasmid free cells can co-grow alongside plasmid containing cells, despite using nutrient selection2,46–48. In our lab we have exploited this property to study metabolite exchange interactions between cells, and developed a system of self-establishing metabolically cooperating communities (SeMeCo) in which a series of auxotrophs cooperate to enable the growth of a yeast community25,45. This system exploits plasmid segregation, starting from an initially self-supporting cell, that grows progressively into an increasingly heterogeneous population, which is able to proliferate on the basis of nutrient exchange occuring between yeast cells. The progressive self-establishment overcomes a failure that is typically observed when yeast auxotrophs are forced to establish a bilateral cooperation. Other than through self-establishment, this lethality is overcome by yeast cells being genetically modified to artificially overproduce metabolites needing to be exchanged. The synthetic communities generated in this way have been intensively studied and serve as a model for ecological metabolite exchange interactions49–52. The new vector series, having multiple auxotrophic markers on single centromeric plasmids, can support the design of such communities, as it removes the likelihood of recombination occurring when multiple plasmids are used in parallel, to obtain the desired auxotrophic background.
Finally, the uniform multiple cloning site (MCS) in the plasmid series allows for the inclusion of marker proteins, such as GFP or beta-galactosidase, to track individual cell types in SeMeCo communities, that reveal phenotypic heterogeneity at the single cell level45. This MCS further allows the use of these plasmids for the recombinant expression of proteins. Here, one can profit from multiple auxotrophic markers on one plasmid to improve selection and reduce plasmid segregation rate, so that (as long as no disadvantageous protein is expressed) the plasmids can be maintained in rich medium in the absence of selection pressure33. This strategy of using multiple markers in parallel can further be exploited to increase selection pressure, to counteract the well-known issue of clonal selection phenotypes, emerging when overexpressing recombinant proteins.
In summary, to test both the effects of prototrophy as well as the metabolic capacity of budding yeast, to design self-establishing metabolically cooperating communities, and to profit from multiple selection markers when expressing proteins, we present a series of centromeric plasmids that can compensate for histidine (HIS3), leucine (LEU2), uracil (URA3), methionine (MET17) or lysine (LYS2) deficiencies in 23 possible combinations. These vectors are accessible individually (Table 2) or as a Kit from Addgene (www.addgene.org/kits/prototrophy/). We hope they benefit the community when analysing the importance of biosynthetic metabolism for gene function, gene expression, physiology and metabolite exchange.
The full sequences of the 23 plasmids are deposited in Addgene under the ID numbers listed in Table 1.
MM designed the plasmids, MM, KC, OM, and FE constructed the plasmids, MM, KC, tested the plasmids, MR designed the study, MM, KC and MR wrote the paper.
This work was supported by the Francis Crick Institute which receives its core funding from Cancer Research UK (FC001134), the UK Medical Research Council (FC001134), and the Wellcome Trust (FC001134), as well as grant funding from the Wellcome Trust (RG 093735/Z/10/Z, 200829/Z/16/Z) and the ERC (Starting Grant 260809) to MR.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Eric Perkins (Addgene) for supporting this project by sequencing of the 23 plasmids.
Plasmid sequences used in Figure 1 and Figure 2. Plasmid sequences for the 23 vectors have been assembled from the pHLUM and pRS300 vector sequences, respectively, and verified and corrected upon resequencing.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 20 Sep 16 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)