Introduction
Alteration in the activity or function of the extracellular Ca2+ (Ca2+ext)-sensing receptor (CaR; also named CaSR or CaS) is linked to several genetic disorders of calcium homeostasis1, such as familial hypocalciuric hypercalcemia (FHH) and neonatal severe hyperparathyroidism (NSHPT)2, both caused by loss-of-function mutations of the CaR gene, and those that occur as a consequence of gain-of function mutations of the CaR, e.g., autosomal dominant hypocalcemia (ADH) and Bartter syndrome (BS) type V3–5. However, the CaR is also a factor in other more common pathologies that include chronic kidney disease6, cancer7, cardiovascular pathologies8–11, and Alzheimer’s disease12. For a complete survey of CaR’s function in molecular physiology and pathology, readers are referred to some of the many recent reviews on the topic13.
We will first address when and how Ca2+ext, the primary ligand for the CaR, changes in tissue spaces. Ca2+ is, however, just one of the many activators of this fascinating receptor; the CaR is “built” to interact with a dizzying array of other orthosteric agonists and also allosteric modulators that influence the receptor’s response to calcium ions (Table 1). These endogenous ligands activate multiple intracellular signaling pathways, often in the same cell type (Figure 1). However, the CaR can discriminate between its ligands to preferentially activate a particular subset of signaling pathways at the exclusion of others through the phenomenon known as biased agonism. In addition, CaR signaling can be dynamically regulated through agonist-dependent trafficking of intracellular receptors to alter the net amount of the receptor at the plasma membrane. We will address how certain ligands act as “pharmacoperones” to shepherd the receptor to the cell surface. All of these factors serve to fine-tune the activity of the receptor. Finally, we discuss the incredible potential of this newfound information to aid in the design of novel, smarter, drugs able to rescue mutated receptor mislocalization and function, and bias CaR-mediated signaling towards particular pathways.
Table 1. Principal orthosteric agonists and allosteric modulators of the calcium-sensing receptor.
Orthosteric agonists
(type I calcimimetics) | | References |
|---|
Inorganic divalent and
trivalent cations | High potency: Gd3+; Eu3+; Tb3+
Intermediate potency: Zn2+; Ni2+; Cd2+; Pb2+; Co2+; Fe2+
Low potency: Ca2+; Mg2+; Ba2+; Sr2+; Mn2+ | 125–128 |
| Polyamines | Spermine, spermidine, putrescine | 129 |
| Aminoglycoside antibiotics | Neomycin, gentamycin, tobramycin, poromomycin,
kanamycin, ribostamycin | 130–132 |
| Basic polypeptides | Poly-l-arginine, poly-l-lysine, protamine, amyloid
β-peptides | 133–135 |
Allosteric modulators
(type II calcimimetics) | | |
| L-amino acids | Phenylalanine, tryptophan, tyrosine, histidine | 136–138 |
| Glutathione analogs | γ-glutamyl-tripeptides: glutathione,
S-methylglutathione,
S-propylglutathione
γ-glutamyl-tripeptides:
γ-Glu-Ala, γ-Glu-Cys | 139–140 |
| Small molecule calcimimetics | First generation:
NPS R-568, NPS R-467 | 141,142 |
| Second generation:
cinacalcet | 143–145 |
| Third generation:
dibenzylamine calcimimetics, R,R-calcimimetic B,
AC-265347 | 94,146,147 |
| Small molecule calcilytics | NPS 2143, Calhex 231, ATF936, AXT914, ronacaleret,
NPSP795, SB-423557, SB-423562 | 97,142,148–150 |
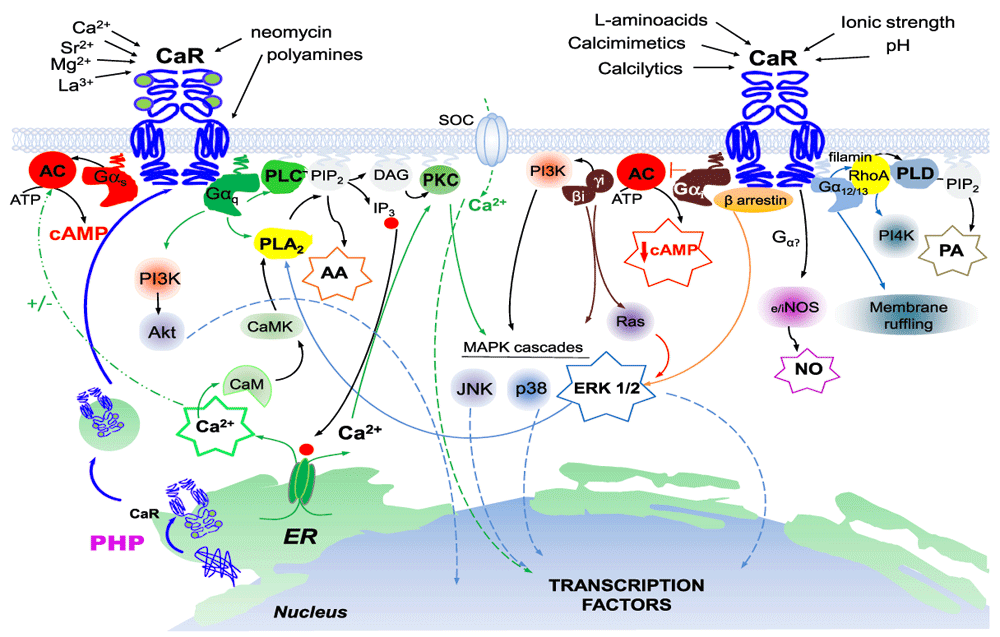
Figure 1. Signal transduction mediated by the extracellular calcium-sensing receptor (CaR).
Schematic of the dimeric extracellular CaR at the plasma membrane. A complex network of intracellular transduction cascades is activated by numerous orthosteric agonists or allosteric modulators converging either on the bi-lobed venus-flytrap domain or on the seven transmembrane domain of the CaR. For clarity, two G-protein-coupled receptors (GPCRs) are shown; this is not meant to imply that the ligands depicted are linked preferentially to a particular intracellular signaling pathway, although see section in text on biased agonism. Abbreviations: AA, arachidonic acid; AC, adenylate cyclase; Akt, protein kinase B; ATP, adenosine triphosphate; CaM, calmodulin; CaMK, Ca2+/calmodulin-dependent protein kinase; cAMP, cyclic AMP; DAG, diacylglycerol; eNOS, endothelial nitric oxide synthase; ER, endoplasmic reticulum; ERK 1/2, extracellullar-signal-regulated kinase; Gαs, Gαi, Gαq, Gα12/13, α subunits of the s-, i-, q-, and 12/13-type heterotrimeric G-proteins, respectively; iNOS, inducible nitric oxide synthase; IP3, inositol-1,4,5-trisphosphate; JNK, Jun amino-terminal kinase; MAPK, mitogen-activated protein kinase; MEK, MAPK kinase; NO, nitric oxide; p38, p38 mitogen-activated protein kinase; PA, phosphatidic acid; PHP, pharmacoperones; PI3K, phosphatidylinositol 3-kinase; PI4K, phosphatidylinositol 4-kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PLA2, phospholipase A2; PLC, phospholipase C; PLD, phospholipase D; RhoA, Ras homolog gene family, member A; SOC, store-operated Ca2+ channel.
Extracellular Ca2+ fluctuations and Ca2+ microdomains
Systemic Ca2+ levels (~1.1–1.3 mM) are under stringent homeostatic control exerted by organs such as the parathyroid glands, bone, renal system, and intestine14. Nonetheless, local fluctuations in Ca2+ext levels have been identified and characterized in the restricted volume of interstitial fluids bathing cells of many tissues15. The amplitude and shape of these Ca2+ext fluctuations is thought to represent an autocrine/paracrine form of cell-to-cell communication. Pharmacological agents directed at the CaR therefore work upon a complex backdrop of changing external [Ca2+]. This has the potential to markedly affect the way in which a drug (particularly those in the class of the allosteric modulators) acts on the receptor in any given moment. Knowledge about these local fluctuations in calcium remains, arguably, among the most significant barriers to fully understanding CaR pharmacology in vivo.
Many factors are believed to participate in the generation of physiologically relevant Ca2+ext changes, e.g. a) intracellular Ca2+ signaling events, b) Ca2+ extrusion via discharge of calcium-enriched granules, and c) synchronous opening of voltage-operated Ca2+ channels. Below, we describe briefly how these extracellular microdomains can be measured and give examples of how they are generated.
Measuring extracellular Ca2+ levels
Historically, real-time measurements of Ca2+ext changes in close proximity to the plasma membrane have been hampered by the lack of proper experimental tools to physically access these restricted compartments in intact tissues and by difficulties in measuring [Ca2+] fluctuations against the background of mM Ca2+ concentrations normally present outside the cell. Although many different experimental approaches have been proposed to quantify Ca2+ext fluctuations in a number of diverse tissue models, each of them presents limitations with regard to either sensitivity or spatial resolution. For example, Ca2+-sensitive small molecule fluorescent indicators have proven useful to visualize the temporal/spatial dynamics of Ca2+ext changes, since they provide sensitivity, time resolution, and access to limited spaces16–24. However, these methods require experiments to be performed in non-physiological conditions such as low or nominally free Ca2+ext because of the relatively high Ca2+ affinity of the available fluorophores. For example, Tepikin and Petersen introduced the droplet technique25–27 to reliably quantify Ca2+ext changes induced by active Ca2+ extrusion through the plasma membrane Ca2+-ATPase (PMCA) of acinar cells. Fluo-3 was used to characterize changes in Ca2+ext in small clusters of exocrine gland cells maintained in a tiny droplet of solution covered with oil to prevent evaporation, but this method could only be used under Ca2+-free media conditions on account of the high affinity of the Ca2+ indicator.
We, as well as others, have used Ca2+-selective microelectrodes extensively to directly record the profile of changes in Ca2+ext in the restricted domains of different experimental tissue models following Ca2+-mobilizing stimulation28–38. As described further below, we also used Ca2+-sensitive microelectrodes to measure real-time Ca2+ext changes induced by the glucose-dependent discharge of Ca2+-rich insulin granules39. Ion-sensitive microelectrodes present certain advantages. First, measurements of Ca2+ex changes under physiological conditions are allowed owing to the availability of Ca2+-sensitive resins with affinities in the μM and mM range. In addition, it is possible to record Ca2+ext changes for hours without technical drawbacks such as the bleaching of fluorescent indicators. However, this approach requires a high level of patience and expertise and samples only one small region of the tissue, so it is not amenable to high-throughput measurements. Moreover, it is difficult to execute in many tissue types. This is an arena in which further developments would be welcome.
Origins of extracellular Ca2+ microdomains
Intracellular Ca2+ signaling events. Cells facing restricted diffusion spaces can experience Ca2+ext fluctuations during intracellular Ca2+ signaling events as a result of activation of Ca2+ efflux (e.g. by PMCA and/or Na+/Ca2+ exchanger) and influx (e.g. by store-operated channels [SOCs]) across the plasma membrane. The genesis of significant Ca2+ext microdomains requires either differential dynamics or polarized asymmetry of Ca2+ influx/efflux mechanisms40–43. For example, we found that stimulation with Ca2+-mobilizing agonists resulted in substantial local increase in Ca2+ext at the luminal face and a comparable depletion at the serosal aspect of gastric acid-secreting cells38. An increase in [Ca2+] in the gastric gland lumen is due to activation of Ca2+-ATPase, which is highly expressed at the apical membrane of these cells, where it co-localizes with CaR38.
Ca2+ extrusion via discharge of calcium-enriched granules. Very high Ca2+ concentrations have been measured within secretory granules44–46. For example, insulin granules from rat insulinoma have a granular concentration of Ca2+ between 60 and 120 mM47. Therefore, one can assume that exocytotic events may generate consistent increases in Ca2+ext. Recently, we showed that the stimulation of insulin secretion by high glucose and other secretagogues resulted in late elevation of Ca2+ext within rat insulinoma (INS-1E) β-cell pseudoislets, as measured with Ca2+ microelectrodes39. Ca2+ extrusion via Ca2+-enriched granules has also been proposed for a number of different cell types that undergo exocytosis such as salivary gland cells22, bovine adrenal medullary cells48, neurohypophyseal nerve endings49, and sea urchin eggs50.
Synchronous opening of voltage-operated Ca2+ channels. Excitable cells have, in addition to the above-mentioned mechanisms, a variety of voltage-dependent Ca2+ entry pathways that might impact Ca2+ext during their physiological activity51–53. In the central nervous system, synchronous opening of voltage-gated Ca2+ channels (VGCCs) can stimulate significant reductions in Ca2+ext54,55. Pumain and Heinemann recorded Ca2+ext reductions from a basal level of 1.25 mM to as low as 0.08 mM in rat neocortex following the application of excitatory amino acids54. In cardiac muscle, transient depletions in Ca2+ext by about 200 μM were measured during a single heartbeat16. In mouse islets of Langerhans and INS-1E pseudoislets, glucose stimulation induced a reversible and significant depletion in Ca2+ext by about 500 μM as a consequence of VGCC-mediated Ca2+ influx across the plasma membrane30,33,39.
New paradigms in extracellular calcium-sensing receptor trafficking and signaling pave the way for the design of novel, smart drugs
In the classical (yet oversimplified) view, upon interaction with a G-protein-coupled receptor (GPCR), ligands stabilize preferred conformational state(s) that in turn activate distinct subsets of G-protein-mediated downstream signaling pathways56–58. When GPCRs are coupled to multiple G-proteins in the same cell type, as is the CaR, the old dogma hypothesized that they activate each of the downstream signals equally, without preference for any one pathway58–61.
In the past few years, several studies have painted a more complex scenario, in which receptors, existing in multiple active states, can specifically trigger selected pathways at the exclusion of others62. This will depend not only on the signaling toolkit of the cell in which they are expressed but also on numerous other factors63, such as the localization of the GPCRs, the duration of stimulus for GPCRs working in non-equilibrium conditions, the downstream signaling protein level (i.e. involvement of different effectors able to shape diverse Ca2+ and cAMP microdomains and kinetics), and the specific agonist/modulator activating the receptor64. Also, it has been shown that GPCRs traffic through subcellular compartments such as the nucleus65, mitochondria66, and endosomes67,68, where they are capable of initiating specific signaling pathways.
In this context, pharmacological studies have shown that ligands are able to bias the signaling of their GPCRs towards specific intracellular responses and/or are capable of crossing cell membranes, thus activating or rescuing intracellular GPCRs (by acting as molecular chaperones)69. The development of new technologies, such as microscopy techniques and probes to follow receptor trafficking63,70 and to assess in real time subcellular signaling dynamics71,72 as well as biased signaling73, has been essential for such advances and will certainly continue to promote novel and exciting discoveries in this field.
The “anti-conformist” extracellular calcium-sensing receptor traffics to the plasma membrane via a novel route: agonist-driven insertional signaling
In the classical life cycle of GPCRs, the newly synthesized receptor is inserted into the endoplasmic reticulum and, after folding, is transported through the cis-Golgi/Golgi/trans-Golgi, where it goes through further post-translational changes. Then the mature protein, packaged in small vesicles, undergoes insertion into the cell membrane. If misfolded, the protein is degraded by the proteasome. Upon binding, ligands stabilize preferred conformational state(s) of the receptor that initiate intracellular signaling. The process is terminated via receptor internalization mediated by GPCR kinase (GRK) phosphorylation and β-arrestin(s) recruitment74. The internalized receptor can be degraded by the lysosome or recycled to the cell membrane. Importantly, both β-arrestin and internalized receptors can initiate signaling.
It is well established that the fine balance among maturation, internalization, recycling, and degradation can influence the net amount of cell surface receptor level and thus represents a mechanism for the cell to regulate receptor sensitization and modulate the strength of signal transduction75. The intensity of signaling is thus related to the quantity of GPCRs expressed on the cell surface and accessible for ligand stimulation. This is also true for the CaR, as recently demonstrated by Brennan and colleagues76.
Relevant advancements in the knowledge of the key players involved in CaR biosynthesis and trafficking have been achieved in the last ten years77–81. Both early and recent studies have highlighted that two hallmarks of the CaR are the negligible functional desensitization and the existence of a significant amount of CaR in intracellular membranes. Early studies indicated, both by western blotting or immunohistochemistry14,82,83, that CaR immunoreactivity reflected a predominantly intracellular, core-glycosylated form. It is now becoming clear that such an observation is not a mere artifact but is strictly related, and even of functional importance, to the complex and mutual interaction between CaR trafficking and signaling. In fact, both minimal desensitization and high levels of intracellular CaR can be explained by the model of agonist-driven insertional signaling (ADIS)78,84.
The process of ADIS depends upon the regulated release of mature CaR proteins from a large intracellular pool located in the endoplasmic reticulum and Golgi/post-Golgi vesicles. The rate of CaR plasma membrane insertion increases as a function of the concentration of CaR agonists and/or allosteric modulators, while the receptor already at the plasma membrane undergoes constitutive endocytosis without substantial recycling. Importantly, and predictably, in this model, CaR signaling can be dynamically regulated by the trafficking of intracellular CaR to the plasma membrane through an agonist-dependent modulation of the net amount of CaR at the plasma membrane. This has implications in both health and disease85.
New insights into the mechanisms underlying the therapeutic potential of allosteric modulators of the extracellular calcium-sensing receptor
As summarized in Table 1, besides the orthosteric ligands, which upon binding to agonist-binding sites are able to stimulate the receptor in the absence of Ca2+ (or any other ligand), the other class of CaR agonists is represented by allosteric modulators, which after binding to different sites alter the receptor conformation and, as a consequence, affect receptor responses to orthosteric ligands. This action can be exerted in a positive (calcimimetics) or a negative (calcilytics [Table 1]) direction.
Interestingly, a number of recent reports have shown that allosteric modulators can act as pharmacoperones. Pharmacoperones (or pharmacological chaperones or pharmacochaperones) are membrane-permeant ligands (agonists, antagonists, or allosteric modulators) that reach the misfolded protein at the site of its biosynthesis and trafficking (most frequently the endoplasmic reticulum) and, by stabilizing the receptor structure, rescue the protein to the cell surface69.
Breitwieser's group has published a number of interesting papers highlighting the capability of CaR allosteric modulators to function as pharmacoperones86–88. While an early study reported the synergistic effect of acute treatment with L-phenylalanine and NPS R-467 on CaRs with inactivating mutations89, Breitwieser and colleagues first showed that overnight treatment of HEK293 cells expressing loss-of-function mutant CaRs with the calcimimetic NPS R-568 rescued plasma membrane expression and signaling in 50% of the mutations examined86,87. Similar results were obtained by other groups, although the authors did not investigate the cell surface expression of CaR after NPS R-568-mediated signal rescue90,91. Interestingly, the capability of the calcimimetic NPS R-568 to rescue CaR activation without altering the cell surface expression of the mutant proteins was shown in a recent study92, suggesting a mutant-specific effect of this drug as a pharmacoperone.
Relevant findings in this area have also been provided by Leach and colleagues93,94. They showed that calcimimetics, including the only calcimimetic approved in the clinic (cinacalcet), effectively rescue trafficking and signaling of CaR mutants exhibiting a loss of cell surface expression. They also found that the calcilytic NPS 2143 effectively promotes trafficking of CaR mutants to the cell membrane while negatively modulating CaR signaling93,95. This is in contrast to other studies with NPS 2143 showing a reduced86 or unchanged92 effect on the expression of diverse CaR gain-of-function mutants, suggesting that a mutant-specific pharmacoperone effect also exists for NPS 2143.
The potential of calcilytics for patients with activating CaR mutations has been further examined in vitro96. More recently, the new quinazolinone-derived calcilytics were shown to be effective in attenuating enhanced calcium signaling in mutations causing BS and ADH97. NPS 2143 was also found to correct signaling defects in HEK293 cells transfected with Gα11-mutated proteins causing ADH2 and uveal melanoma98. Very interestingly, the effectiveness of both old (i.e. NPS 2143)99 and new (i.e. JTT-305/MK-5442)100 calcilytics was recently assessed in vivo in mouse models harboring ADH gain-of-function CaR mutations.
Biased signaling at the extracellular calcium-sensing receptor
Recent reports suggest that the therapeutic potential of new classes of CaR modulators, as well as the pathophysiological role of endogenous agonists, could be further improved by exploiting the phenomenon of biased signaling11. Biased signaling (also known as ligand-directed signaling, stimulus bias, biased agonism, or functional selectivity)62,101,102 represents a general, albeit only recently appreciated, signaling characteristic of GPCRs58. It refers to the ability of different ligands to stabilize distinct receptor conformations and preferentially direct GPCR signaling towards a specific set of pathways while excluding/reducing others.
This concept, while greatly complicating the scenario of GPCR signaling, opens up new perspectives in the design of smart and tissue-specific drugs103. The existence of ligand- and tissue-specific effects in the signaling pathways activated by the CaR, although not precisely quantified, is traceable in a vast number of papers published throughout the years. In fact, in many cases, biased signaling at the CaR might have been underestimated owing to the use of single assays for the evaluation of CaR signaling outputs (most commonly cytosolic calcium dynamics) or the low number of CaR agonists and modulators tested.
A peculiar case of biased signaling at the CaR was observed in response to an allosteric autoantibody isolated from a patient with acquired hypocalciuric hypocalcemia. The antibody potentiated the effects of Ca2+ext via Gq signaling while suppressing Gi-mediated signaling104. In other examples, Bruce and colleagues reported differential effects of CaR agonists on Ca2+ dynamics in isolated acini and interlobular ducts of rat pancreas105. Ziegelstein showed that in human aortic endothelial cells only spermine was able to induce intracellular Ca2+ release and nitric oxide production, whereas Ca2+ext, Gd3+, and neomycin were ineffective106. Furthermore, Smajilovic et al. demonstrated a concentration-dependent vasodilatation in rat aorta with the addition of cinacalcet, whereas the agonists neomycin and Gd3+ were ineffective107.
On these bases, in the last three years, an increasing number of reports have focused on the physiological and pathological role of biased signaling exerted on the CaR by its physiological agonists and pharmacological modulators as well as on mutation-dependent alterations in such bias. A key contribution to this field comes from the group of Bräuner-Osborne108–110. By exploring the effect of 12 orthosteric CaR agonists on inositol (1,4,5)-trisphosphate (IP3) accumulation, cAMP inhibition, and ERK1/2 phosphorylation in HEK293 cells stably transfected with rat CaR, Thomsen and colleagues110 revealed that Ca2+ext is biased towards cAMP inhibition and IP3 accumulation, while spermine shows a strong bias towards ERK1/2 phosphorylation. Also, this study demonstrated for the first time that ERK1/2 is partially activated through the recruitment of β-arrestin by the CaR. The same group also obtained interesting results concerning strontium ranelate, currently used in the clinic for the treatment of osteoporosis109. As previously suggested by Chattopadhyay and colleagues111, and contrary to the results obtained by Coulombe112, Sr2+ was shown to bias CaR signaling towards ERK1/2 in rat medullary thyroid carcinoma 6–23 cells. Also, in rabbit osteoclasts, while both Sr2+ and Ca2+ produced stimulation of PLC and translocation of NF-kB, in contrast to Ca2+ext, Sr2+ signaling was independent of the IP3 pathway and induced apoptosis via PKC activation113.
The possibility of exploiting biased agonism at the CaR has been extensively explored by the group of Christopoulos and Leach93,114–116. These authors analyzed the effect of calcimimetics and calcilytics on a number of CaR mutations115 (reviewed in 95,103), demonstrating that mutated CaR proteins can display altered signaling bias. Importantly, and as stated above, both cinacalcet and NPS 2143 were shown to effectively rescue mutants to the cell membrane, with a bias of both compounds toward the modulation of agonist-stimulated Ca2+ mobilization93. There is no doubt that these results have relevant therapeutic potential.
To date, cinacalcet has been used for the treatment of hyperparathyroidism and to correct Ca2+ext in patients with loss-of-function CaR mutations. However, because of its hypocalcemic side effects, presumably due to CaR-mediated calcium-dependent calcitonin secretion from thyroid parafollicular C-cells108 and potentiation of renal CaRs, its use has been restricted to patients with end-stage renal disease. Thus, a drug that suppresses PTH secretion without raising serum calcitonin would be therapeutically advantageous.
Potential clues towards the search for a calcimimetic with low/no effect on calcitonin was hinted at in a very recent paper94. In this work, the authors demonstrated that while phenylalkylamine calcimimetics were biased towards Ca2+ mobilization and IP1 accumulation (a stable metabolite of IP3), R,R-calcimimetic B and AC-265347 biased CaR signaling towards pERK1/2 and IP1 accumulation. This finding may explain the preference of R,R-calcimimetic B and AC-265347 for the suppression of PTH release versus the stimulation of calcitonin secretion in vivo.
Structure-function relationships and future prospects
Recent work explored the structural requirements for bias and allostery mediated by old and new classes of positive and negative allosteric modulators of the CaR116. Further, Jenny Yang’s lab has published several papers about the potential Ca2+ binding sites and their relevance for related diseases117–124. Recently, these authors solved the first high-resolution crystal structure of the ECD of human CaR bound with Mg2+117. Of note, a high-affinity tryptophan derivative was found in the crystal structure of the CaR that seems to play a role in potentiating the function of the receptor117. These studies represent important progress in the field, since they provide new insights into the structural basis of human diseases arising from CaR mutations. Ultimately, the subtle differences in modulator binding sites revealed by structural studies may be exploited to design drugs able to elicit distinct signaling outcomes and thus be effective on specific mutations (patient-specific drugs) and/or on tissue-specific signaling pathways (tissue-specific drugs).
In this scenario, a fundamental challenge for future research will be to set up methodological tools to validate these latest pharmacological advances in more physiologically relevant models, such as primary cells or animal models. It also remains to be seen how the functional effects of these drugs are altered in the complex landscape of changing [Ca2+] in extracellular microdomains in vivo.
Author contributions
Matilde Colella and Andrea Gerbino contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Grant information
The author(s) declared that no grants were involved in supporting this work.
Faculty Opinions recommendedReferences
- 1.
Brown EM:
Mutations in the calcium-sensing receptor and their clinical implications.
Horm Res.
1997; 48(5): 199–208. PubMed Abstract
| Publisher Full Text
- 2.
Pollak MR, Brown EM, Chou YH, et al.:
Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism.
Cell.
1993; 75(7): 1297–303. PubMed Abstract
| Publisher Full Text
- 3.
Watanabe S, Fukumoto S, Chang H, et al.:
Association between activating mutations of calcium-sensing receptor and Bartter's syndrome.
Lancet.
2002; 360(9334): 692–4. PubMed Abstract
| Publisher Full Text
- 4.
Pollak MR, Brown EM, Estep HL, et al.:
Autosomal dominant hypocalcaemia caused by a Ca2+-sensing receptor gene mutation.
Nat Genet.
1994; 8(3): 303–7. PubMed Abstract
| Publisher Full Text
- 5.
Zhao XM, Hauache O, Goldsmith PK, et al.:
A missense mutation in the seventh transmembrane domain constitutively activates the human Ca2+ receptor.
FEBS Lett.
1999; 448(1): 180–4. PubMed Abstract
| Publisher Full Text
- 6.
Massy ZA, Hénaut L, Larsson TE, et al.:
Calcium-sensing receptor activation in chronic kidney disease: effects beyond parathyroid hormone control.
Semin Nephrol.
2014; 34(6): 648–59. PubMed Abstract
| Publisher Full Text
- 7.
Tennakoon S, Aggarwal A, Kállay E:
The calcium-sensing receptor and the hallmarks of cancer.
Biochim Biophys Acta.
2016; 1863(6 Pt B): 1398–407. PubMed Abstract
| Publisher Full Text
- 8.
Smajilovic S, Yano S, Jabbari R, et al.:
The calcium-sensing receptor and calcimimetics in blood pressure modulation.
Br J Pharmacol.
2011; 164(3): 884–93. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 9.
Weston AH, Geraghty A, Egner I, et al.:
The vascular extracellular calcium-sensing receptor: an update.
Acta Physiol (Oxf).
2011; 203(1): 127–37. PubMed Abstract
| Publisher Full Text
- 10.
Toka HR, Pollak MR:
The role of the calcium-sensing receptor in disorders of abnormal calcium handling and cardiovascular disease.
Curr Opin Nephrol Hypertens.
2014; 23(5): 494–501. PubMed Abstract
| Publisher Full Text
- 11.
Schepelmann M, Yarova PL, Lopez-Fernandez I, et al.:
The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure.
Am J Physiol Cell Physiol.
2016; 310(3): C193–204. PubMed Abstract
| Publisher Full Text
- 12.
Dal Prà I, Chiarini A, Armato U:
Antagonizing amyloid-β/calcium-sensing receptor signaling in human astrocytes and neurons: a key to halt Alzheimer's disease progression?
Neural Regen Res.
2015; 10(2): 213–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 13.
Brown EM, Conigrave AD:
Preface.
Best Pract Res Clin Endocrinol Metab.
2013; 27(3): 283–4. PubMed Abstract
| Publisher Full Text
- 14.
Brown EM, MacLeod RJ:
Extracellular calcium sensing and extracellular calcium signaling.
Physiol Rev.
2001; 81(1): 239–97. PubMed Abstract
- 15.
Hofer AM:
Another dimension to calcium signaling: a look at extracellular calcium.
J Cell Sci.
2005; 118(Pt 5): 855–62. PubMed Abstract
| Publisher Full Text
- 16.
Cleemann L, Pizarro G, Morad M:
Optical measurements of extracellular calcium depletion during a single heartbeat.
Science.
1984; 226(4671): 174–7. PubMed Abstract
| Publisher Full Text
- 17.
Hilgemann DW, Langer GA:
Transsarcolemmal calcium movements in arterially perfused rabbit right ventricle measured with extracellular calcium-sensitive dyes.
Circ Res.
1984; 54(4): 461–7. PubMed Abstract
| Publisher Full Text
- 18.
Hilgemann DW:
Extracellular calcium transients at single excitations in rabbit atrium measured with tetramethylmurexide.
J Gen Physiol.
1986; 87(5): 707–35. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 19.
Etter EF, Kuhn MA, Fay FS:
Detection of changes in near-membrane Ca2+ concentration using a novel membrane-associated Ca2+ indicator.
J Biol Chem.
1994; 269(13): 10141–9. PubMed Abstract
- 20.
Etter EF, Minta A, Poenie M, et al.:
Near-membrane [Ca2+] transients resolved using the Ca2+ indicator FFP18.
Proc Natl Acad Sci U S A.
1996; 93(11): 5368–73. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 21.
Belan PV, Gerasimenko OV, Tepikin AV, et al.:
Localization of Ca2+ extrusion sites in pancreatic acinar cells.
J Biol Chem.
1996; 271(13): 7615–9. PubMed Abstract
| Publisher Full Text
- 22.
Belan P, Gardner J, Gerasimenko O, et al.:
Isoproterenol evokes extracellular Ca2+ spikes due to secretory events in salivary gland cells.
J Biol Chem.
1998; 273(7): 4106–11. PubMed Abstract
| Publisher Full Text
- 23.
Blatter LA, Niggli E:
Confocal near-membrane detection of calcium in cardiac myocytes.
Cell Calcium.
1998; 23(5): 269–79. PubMed Abstract
| Publisher Full Text
- 24.
De Luisi A, Hofer AM:
Evidence that Ca2+ cycling by the plasma membrane Ca2+-ATPase increases the 'excitability' of the extracellular Ca2+-sensing receptor.
J Cell Sci.
2003; 116(Pt 8): 1527–38. PubMed Abstract
| Publisher Full Text
- 25.
Tepikin AV, Voronina SG, Gallacher DV, et al.:
Acetylcholine-evoked increase in the cytoplasmic Ca2+ concentration and Ca2+ extrusion measured simultaneously in single mouse pancreatic acinar cells.
J Biol Chem.
1992; 267(6): 3569–72. PubMed Abstract
- 26.
Tepikin AV, Voronina SG, Gallacher DV, et al.:
Pulsatile Ca2+ extrusion from single pancreatic acinar cells during receptor-activated cytosolic Ca2+ spiking.
J Biol Chem.
1992; 267(20): 14073–6. PubMed Abstract
- 27.
Tepikin AV, Llopis J, Snitsarev VA, et al.:
The droplet technique: measurement of calcium extrusion from single isolated mammalian cells.
Pflugers Arch.
1994; 428(5–6): 664–70. PubMed Abstract
| Publisher Full Text
- 28.
Jaffe LF, Nuccitelli R:
An ultrasensitive vibrating probe for measuring steady extracellular currents.
J Cell Biol.
1974; 63(2 Pt 1): 614–28. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 29.
Nicholson C, Bruggencate GT, Steinberg R, et al.:
Calcium modulation in brain extracellular microenvironment demonstrated with ion-selective micropipette.
Proc Natl Acad Sci U S A.
1977; 74(3): 1287–90. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 30.
Perez-Armendariz E, Atwater I:
Glucose-evoked changes in [K+] and [Ca2+] in the intercellular spaces of the mouse islet of Langerhans.
Adv Exp Med Biol.
1986; 211: 31–51. PubMed Abstract
| Publisher Full Text
- 31.
Kuhtreiber WM, Jaffe LF:
Detection of extracellular calcium gradients with a calcium-specific vibrating electrode.
J Cell Biol.
1990; 110(5): 1565–73. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 32.
Smith PJ, Sanger RH, Jaffe LF:
The vibrating Ca2+ electrode: a new technique for detecting plasma membrane regions of Ca2+ influx and efflux.
Methods Cell Biol.
1994; 40: 115–34. PubMed Abstract
- 33.
Moura AS:
Membrane potential and intercellular calcium during glucose challenge in mouse islet of Langerhans.
Biochem Biophys Res Commun.
1995; 214(3): 798–802. PubMed Abstract
| Publisher Full Text
- 34.
Knox RJ, Jonas EA, Kao LS, et al.:
Ca2+ influx and activation of a cation current are coupled to intracellular Ca2+ release in peptidergic neurons of Aplysia californica.
J Physiol.
1996; 494(Pt 3): 627–39. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 35.
Yamoah EN, Lumpkin EA, Dumont RA, et al.:
Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia.
J Neurosci.
1998; 18(2): 610–24. PubMed Abstract
- 36.
Pepperell JR, Kommineni K, Buradagunta S, et al.:
Transmembrane regulation of intracellular calcium by a plasma membrane sodium/calcium exchanger in mouse ova.
Biol Reprod.
1999; 60(5): 1137–43. PubMed Abstract
| Publisher Full Text
- 37.
Smith PJ, Hammar K, Porterfield DM, et al.:
Self-referencing, non-invasive, ion selective electrode for single cell detection of trans-plasma membrane calcium flux.
Microsc Res Tech.
1999; 46(6): 398–417. PubMed Abstract
| Publisher Full Text
- 38.
Caroppo R, Gerbino A, Debellis L, et al.:
Asymmetrical, agonist-induced fluctuations in local extracellular [Ca2+] in intact polarized epithelia.
EMBO J.
2001; 20(22): 6316–26. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 39.
Gerbino A, Maiellaro I, Carmone C, et al.:
Glucose increases extracellular [Ca2+] in rat insulinoma (INS-1E) pseudoislets as measured with Ca2+-sensitive microelectrodes.
Cell Calcium.
2012; 51(5): 393–401. PubMed Abstract
| Publisher Full Text
- 40.
Belan P, Gerasimenko O, Petersen OH, et al.:
Distribution of Ca2+ extrusion sites on the mouse pancreatic acinar cell surface.
Cell Calcium.
1997; 22(1): 5–10. PubMed Abstract
| Publisher Full Text
- 41.
Ashby MC, Tepikin AV:
Polarized calcium and calmodulin signaling in secretory epithelia.
Physiol Rev.
2002; 82(3): 701–34. PubMed Abstract
| Publisher Full Text
- 42.
Peng J, Brown EM, Hediger MA:
Apical entry channels in calcium-transporting epithelia.
News Physiol Sci.
2003; 18(4): 158–63. PubMed Abstract
| Publisher Full Text
- 43.
Petersen OH:
Localization and regulation of Ca2+ entry and exit pathways in exocrine gland cells.
Cell Calcium.
2003; 33(5–6): 337–44. PubMed Abstract
| Publisher Full Text
- 44.
Andersson T, Berggren PO, Gylfe E, et al.:
Amounts and distribution of intracellular magnesium and calcium in pancreatic beta-cells.
Acta Physiol Scand.
1982; 114(2): 235–41. PubMed Abstract
| Publisher Full Text
- 45.
Gillot I, Ciapa B, Payan P, et al.:
The calcium content of cortical granules and the loss of calcium from sea urchin eggs at fertilization.
Dev Biol.
1991; 146(2): 396–405. PubMed Abstract
| Publisher Full Text
- 46.
Nicaise G, Maggio K, Thirion S, et al.:
The calcium loading of secretory granules. A possible key event in stimulus-secretion coupling.
Biol Cell.
1992; 75(2): 89–99. PubMed Abstract
| Publisher Full Text
- 47.
Hutton JC, Penn EJ, Peshavaria M:
Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate.
Biochem J.
1983; 210(2): 297–305. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 48.
von Grafenstein HR, Powis DA:
Calcium is released by exocytosis together with catecholamines from bovine adrenal medullary cells.
J Neurochem.
1989; 53(2): 428–35. PubMed Abstract
| Publisher Full Text
- 49.
Thirion S, Stuenkel EL, Nicaise G:
Calcium loading of secretory granules in stimulated neurohypophysial nerve endings.
Neuroscience.
1995; 64(1): 125–37. PubMed Abstract
| Publisher Full Text
- 50.
Kuhtreiber WM, Gillot I, Sardet C, et al.:
Net calcium and acid release at fertilization in eggs of sea urchins and ascidians.
Cell Calcium.
1993; 14(1): 73–86. PubMed Abstract
| Publisher Full Text
- 51.
Vassilev PM, Mitchel J, Vassilev M, et al.:
Assessment of frequency-dependent alterations in the level of extracellular Ca2+ in the synaptic cleft.
Biophys J.
1997; 72(5): 2103–16. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 52.
Egelman DM, Montague PR:
Calcium dynamics in the extracellular space of mammalian neural tissue.
Biophys J.
1999; 76(4): 1856–67. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 53.
Cohen JE, Fields RD:
Extracellular calcium depletion in synaptic transmission.
Neuroscientist.
2004; 10(1): 12–7. PubMed Abstract
| Publisher Full Text
- 54.
Pumain R, Heinemann U:
Stimulus- and amino acid-induced calcium and potassium changes in rat neocortex.
J Neurophysiol.
1985; 53(1): 1–16. PubMed Abstract
- 55.
Rusakov DA, Fine A:
Extracellular Ca2+ depletion contributes to fast activity-dependent modulation of synaptic transmission in the brain.
Neuron.
2003; 37(2): 287–97. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 56.
Wess J:
G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition.
FASEB J.
1997; 11(5): 346–54. PubMed Abstract
- 57.
Lefkowitz RJ:
Historical review: a brief history and personal retrospective of seven-transmembrane receptors.
Trends Pharmacol Sci.
2004; 25(8): 413–22. PubMed Abstract
| Publisher Full Text
- 58.
Rajagopal S, Ahn S, Rominger DH, et al.:
Quantifying ligand bias at seven-transmembrane receptors.
Mol Pharmacol.
2011; 80(3): 367–77. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 59.
Benovic JL, Staniszewski C, Mayor F, et al.:
beta-Adrenergic receptor kinase. Activity of partial agonists for stimulation of adenylate cyclase correlates with ability to promote receptor phosphorylation.
J Biol Chem.
1988; 263(8): 3893–7. PubMed Abstract
- 60.
Kenakin T, Christopoulos A:
Signalling bias in new drug discovery: detection, quantification and therapeutic impact.
Nat Rev Drug Discov.
2013; 12(3): 205–16. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 61.
Makita N, Iiri T:
Biased agonism: a novel paradigm in G protein-coupled receptor signaling observed in acquired hypocalciuric hypercalcemia.
Endocr J.
2014; 61(4): 303–9. PubMed Abstract
| Publisher Full Text
- 62.
Lefkowitz RJ:
A brief history of G-protein coupled receptors (Nobel Lecture).
Angew Chem Int Ed Engl.
2013; 52(25): 6366–78. PubMed Abstract
| Publisher Full Text
- 63.
Lohse MJ, Hofmann KP:
Spatial and Temporal Aspects of Signaling by G-Protein–Coupled Receptors.
Mol Pharmacol.
2015; 88(3): 572–8. PubMed Abstract
| Publisher Full Text
- 64.
Pupo AS, Duarte DA, Lima V, et al.:
Recent updates on GPCR biased agonism.
Pharmacol Res.
2016; 112: 49–57. PubMed Abstract
| Publisher Full Text
- 65.
Ibarra C, Vicencio JM, Estrada M, et al.:
Local control of nuclear calcium signaling in cardiac myocytes by perinuclear microdomains of sarcolemmal insulin-like growth factor 1 receptors.
Circ Res.
2013; 112(2): 236–45. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 66.
Bénard G, Massa F, Puente N, et al.:
Mitochondrial CB1 receptors regulate neuronal energy metabolism.
Nat Neurosci.
2012; 15(4): 558–64. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 67.
Calebiro D, Nikolaev VO, Gagliani MC, et al.:
Persistent cAMP-signals triggered by internalized G-protein–coupled receptors.
PLoS Biol.
2009; 7(8): e1000172. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 68.
Irannejad R, Tomshine JC, Tomshine JR, et al.:
Conformational biosensors reveal GPCR signalling from endosomes.
Nature.
2013; 495(7442): 534–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 69.
Tao YX, Conn PM:
Chaperoning G protein-coupled receptors: from cell biology to therapeutics.
Endocr Rev.
2014; 35(4): 602–47. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 70.
Calebiro D, Sungkaworn T, Maiellaro I:
Real-time monitoring of GPCR/cAMP signalling by FRET and single-molecule microscopy.
Horm Metab Res.
2014; 46(12): 827–32. PubMed Abstract
| Publisher Full Text
- 71.
Miyawaki A, Jaffrey SR:
Editorial overview: Molecular imaging: Cellular imaging approaches.
Curr Opin Chem Biol.
2015; 27: v–vi. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 72.
Karunarathne WK, O'Neill PR, Gautam N:
Subcellular optogenetics - controlling signaling and single-cell behavior.
J Cell Sci.
2015; 128(1): 15–25. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 73.
Johnstone EK, Pfleger KD:
Bioluminescence Resonance Energy Transfer Approaches to Discover Bias in GPCR Signaling.
Methods Mol Biol.
2015; 1335: 191–204. PubMed Abstract
| Publisher Full Text
- 74.
Lefkowitz RJ:
Arrestins come of age: a personal historical perspective.
Prog Mol Biol Transl Sci.
2013; 118: 3–18. PubMed Abstract
| Publisher Full Text
- 75.
Holtbäck U, Brismar H, DiBona GF, et al.:
Receptor recruitment: a mechanism for interactions between G protein-coupled receptors.
Proc Natl Acad Sci U S A.
1999; 96(13): 7271–5. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 76.
Brennan SC, Mun H, Leach K, et al.:
Receptor expression modulates calcium-sensing receptor mediated intracellular Ca2+ mobilization.
Endocrinology.
2015; 156(4): 1330–42. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 77.
Huang C, Miller RT:
The calcium-sensing receptor and its interacting proteins.
J Cell Mol Med.
2007; 11(5): 923–34. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 78.
Breitwieser GE:
Minireview: the intimate link between calcium sensing receptor trafficking and signaling: implications for disorders of calcium homeostasis.
Mol Endocrinol.
2012; 26(9): 1482–95. PubMed Abstract
| Publisher Full Text
- 79.
Breitwieser GE:
The calcium sensing receptor life cycle: trafficking, cell surface expression, and degradation.
Best Pract Res Clin Endocrinol Metab.
2013; 27(3): 303–13. PubMed Abstract
| Publisher Full Text
- 80.
Breitwieser GE:
Pharmacoperones and the calcium sensing receptor: exogenous and endogenous regulators.
Pharmacol Res.
2014; 83: 30–7. PubMed Abstract
| Publisher Full Text
- 81.
Ray K:
Calcium-Sensing Receptor: Trafficking, Endocytosis, Recycling, and Importance of Interacting Proteins.
Prog Mol Biol Transl Sci.
2015; 132: 127–50. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 82.
Bai M, Quinn S, Trivedi S, et al.:
Expression and characterization of inactivating and activating mutations in the human Ca2+o-sensing receptor.
J Biol Chem.
1996; 271(32): 19537–45. PubMed Abstract
| Publisher Full Text
- 83.
Bai M, Trivedi S, Brown EM:
Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells.
J Biol Chem.
1998; 273(36): 23605–10. PubMed Abstract
| Publisher Full Text
- 84.
Grant MP, Stepanchick A, Cavanaugh A, et al.:
Agonist-driven maturation and plasma membrane insertion of calcium-sensing receptors dynamically control signal amplitude.
Sci Signal.
2011; 4(200): ra78. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 85.
Grant MP, Stepanchick A, Breitwieser GE:
Calcium signaling regulates trafficking of familial hypocalciuric hypercalcemia (FHH) mutants of the calcium sensing receptor.
Mol Endocrinol.
2012; 26(12): 2081–91. PubMed Abstract
| Publisher Full Text
- 86.
Huang Y, Breitwieser GE:
Rescue of calcium-sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis.
J Biol Chem.
2007; 282(13): 9517–25. PubMed Abstract
| Publisher Full Text
- 87.
White E, McKenna J, Cavanaugh A, et al.:
Pharmacochaperone-mediated rescue of calcium-sensing receptor loss-of-function mutants.
Mol Endocrinol.
2009; 23(7): 1115–23. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 88.
Cavanaugh A, McKenna J, Stepanchick A, et al.:
Calcium-sensing receptor biosynthesis includes a cotranslational conformational checkpoint and endoplasmic reticulum retention.
J Biol Chem.
2010; 285(26): 19854–64. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 89.
Zhang Z, Jiang Y, Quinn SJ, et al.:
L-phenylalanine and NPS R-467 synergistically potentiate the function of the extracellular calcium-sensing receptor through distinct sites.
J Biol Chem.
2002; 277(37): 33736–41. PubMed Abstract
| Publisher Full Text
- 90.
Rus R, Haag C, Bumke-Vogt C, et al.:
Novel inactivating mutations of the calcium-sensing receptor: the calcimimetic NPS R-568 improves signal transduction of mutant receptors.
J Clin Endocrinol Metab.
2008; 93(12): 4797–803. PubMed Abstract
| Publisher Full Text
- 91.
Lu JY, Yang Y, Gnacadja G, et al.:
Effect of the calcimimetic R-568 3-(2-chlorophenyl)-N-((1R)-1-[(3-methoxyphenyl)ethyl)-1-propanamine] on correcting inactivating mutations in the human calcium-sensing receptor.
J Pharmacol Exp Ther.
2009; 331(3): 775–86. PubMed Abstract
| Publisher Full Text
- 92.
Nakamura A, Hotsubo T, Kobayashi K, et al.:
Loss-of-function and gain-of-function mutations of calcium-sensing receptor: functional analysis and the effect of allosteric modulators NPS R-568 and NPS 2143.
J Clin Endocrinol Metab.
2013; 98(10): E1692–701. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 93.
Leach K, Wen A, Cook AE, et al.:
Impact of clinically relevant mutations on the pharmacoregulation and signaling bias of the calcium-sensing receptor by positive and negative allosteric modulators.
Endocrinology.
2013; 154(3): 1105–16. PubMed Abstract
| Publisher Full Text
- 94.
Cook AE, Mistry SN, Gregory KJ, et al.:
Biased allosteric modulation at the CaS receptor engendered by structurally diverse calcimimetics.
Br J Pharmacol.
2015; 172(1): 185–200. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 95.
Leach K, Sexton PM, Christopoulos A, et al.:
Engendering biased signalling from the calcium-sensing receptor for the pharmacotherapy of diverse disorders.
Br J Pharmacol.
2014; 171(5): 1142–55. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 96.
Letz S, Rus R, Haag C, et al.:
Novel activating mutations of the calcium-sensing receptor: the calcilytic NPS-2143 mitigates excessive signal transduction of mutant receptors.
J Clin Endocrinol Metab.
2010; 95(10): E229–33. PubMed Abstract
| Publisher Full Text
- 97.
Letz S, Haag C, Schulze E, et al.:
Amino alcohol- (NPS-2143) and quinazolinone-derived calcilytics (ATF936 and AXT914) differentially mitigate excessive signalling of calcium-sensing receptor mutants causing Bartter syndrome Type 5 and autosomal dominant hypocalcemia.
PLoS One.
2014; 9(12): e115178. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 98.
Babinsky VN, Hannan FM, Gorvin CM, et al.:
Allosteric Modulation of the Calcium-sensing Receptor Rectifies Signaling Abnormalities Associated with G-protein α-11 Mutations Causing Hypercalcemic and Hypocalcemic Disorders.
J Biol Chem.
2016; 291(20): 10876–85. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 99.
Hannan FM, Walls GV, Babinsky VN, et al.:
The Calcilytic Agent NPS 2143 Rectifies Hypocalcemia in a Mouse Model With an Activating Calcium-Sensing Receptor (CaSR) Mutation: Relevance to Autosomal Dominant Hypocalcemia Type 1 (ADH1).
Endocrinology.
2015; 156(9): 3114–21. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 100.
Dong B, Endo I, Ohnishi Y, et al.:
Calcilytic Ameliorates Abnormalities of Mutant Calcium-Sensing Receptor (CaSR) Knock-In Mice Mimicking Autosomal Dominant Hypocalcemia (ADH).
J Bone Miner Res.
2015; 30(11): 1980–93. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 101.
Kenakin T:
Agonist-receptor efficacy. I: Mechanisms of efficacy and receptor promiscuity.
Trends Pharmacol Sci.
1995; 16(6): 188–92. PubMed Abstract
| Publisher Full Text
- 102.
Kenakin T:
Agonist-receptor efficacy. II. Agonist trafficking of receptor signals.
Trends Pharmacol Sci.
1995; 16(7): 232–8. PubMed Abstract
| Publisher Full Text
- 103.
Leach K, Conigrave AD, Sexton PM, et al.:
Towards tissue-specific pharmacology: insights from the calcium-sensing receptor as a paradigm for GPCR (patho)physiological bias.
Trends Pharmacol Sci.
2015; 36(4): 215–25. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 104.
Makita N, Sato J, Manaka K, et al.:
An acquired hypocalciuric hypercalcemia autoantibody induces allosteric transition among active human Ca-sensing receptor conformations.
Proc Natl Acad Sci U S A.
2007; 104(13): 5443–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 105.
Bruce JI, Yang X, Ferguson CJ, et al.:
Molecular and functional identification of a Ca2+ (polyvalent cation)-sensing receptor in rat pancreas.
J Biol Chem.
1999; 274(29): 20561–8. PubMed Abstract
| Publisher Full Text
- 106.
Ziegelstein RC, Xiong Y, He C, et al.:
Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells.
Biochem Biophys Res Commun.
2006; 342(1): 153–63. PubMed Abstract
| Publisher Full Text
- 107.
Smajilovic S, Sheykhzade M, Holmegard HN, et al.:
Calcimimetic, AMG 073, induces relaxation on isolated rat aorta.
Vascul Pharmacol.
2007; 47(4): 222–8. PubMed Abstract
| Publisher Full Text
- 108.
Thomsen AR, Smajilovic S, Bräuner-Osborne H:
Novel strategies in drug discovery of the calcium-sensing receptor based on biased signaling.
Curr Drug Targets.
2012; 13(10): 1324–35. PubMed Abstract
| Publisher Full Text
- 109.
Thomsen AR, Worm J, Jacobsen SE, et al.:
Strontium is a biased agonist of the calcium-sensing receptor in rat medullary thyroid carcinoma 6-23 cells.
J Pharmacol Exp Ther.
2012; 343(3): 638–49. PubMed Abstract
| Publisher Full Text
- 110.
Thomsen AR, Hvidtfeldt M, Bräuner-Osborne H:
Biased agonism of the calcium-sensing receptor.
Cell Calcium.
2012; 51(2): 107–16. PubMed Abstract
| Publisher Full Text
| Faculty Opinions Recommendation
- 111.
Chattopadhyay N, Quinn SJ, Kifor O, et al.:
The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation.
Biochem Pharmacol.
2007; 74(3): 438–47. PubMed Abstract
| Publisher Full Text
- 112.
Coulombe J, Faure H, Robin B, et al.:
In vitro effects of strontium ranelate on the extracellular calcium-sensing receptor.
Biochem Biophys Res Commun.
2004; 323(4): 1184–90. PubMed Abstract
| Publisher Full Text
- 113.
Hurtel-Lemaire AS, Mentaverri R, Caudrillier A, et al.:
The calcium-sensing receptor is involved in strontium ranelate-induced osteoclast apoptosis. New insights into the associated signaling pathways.
J Biol Chem.
2009; 284(1): 575–84. PubMed Abstract
| Publisher Full Text
- 114.
Davey AE, Leach K, Valant C, et al.:
Positive and negative allosteric modulators promote biased signaling at the calcium-sensing receptor.
Endocrinology.
2012; 153(3): 1232–41. PubMed Abstract
| Publisher Full Text
- 115.
Leach K, Wen A, Davey AE, et al.:
Identification of molecular phenotypes and biased signaling induced by naturally occurring mutations of the human calcium-sensing receptor.
Endocrinology.
2012; 153(9): 4304–16. PubMed Abstract
| Publisher Full Text
- 116.
Leach K, Gregory KJ, Kufareva I, et al.:
Towards a structural understanding of allosteric drugs at the human calcium-sensing receptor.
Cell Res.
2016; 26(5): 574–92. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 117.
Zhang C, Zhang T, Zou J, et al.:
Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist.
Sci Adv.
2016; 2(5): e1600241. PubMed Abstract
| Publisher Full Text
| Free Full Text
| Faculty Opinions Recommendation
- 118.
Zhang C, Miller CL, Brown EM, et al.:
The calcium sensing receptor: from calcium sensing to signaling.
Sci China Life Sci.
2015; 58(1): 14–27. PubMed Abstract
| Publisher Full Text
- 119.
Zhang C, Zhuo Y, Moniz HA, et al.:
Direct determination of multiple ligand interactions with the extracellular domain of the calcium-sensing receptor.
J Biol Chem.
2014; 289(48): 33529–42. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 120.
Zhang C, Huang Y, Jiang Y, et al.:
Identification of an L-phenylalanine binding site enhancing the cooperative responses of the calcium-sensing receptor to calcium.
J Biol Chem.
2014; 289(8): 5296–309. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 121.
Zhang C, Mulpuri N, Hannan FM, et al.:
Role of Ca2+ and L-Phe in regulating functional cooperativity of disease-associated "toggle" calcium-sensing receptor mutations.
PLoS One.
2014; 9(11): e113622. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 122.
Huang Y, Zhou Y, Wong H, et al.:
Calmodulin regulates Ca2+-sensing receptor-mediated Ca2+ signaling and its cell surface expression.
J Biol Chem.
2010; 285(46): 35919–31. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 123.
Huang Y, Zhou Y, Castiblanco A, et al.:
Multiple Ca2+-binding sites in the extracellular domain of the Ca2+-sensing receptor corresponding to cooperative Ca2+ response.
Biochemistry.
2009; 48(2): 388–98. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 124.
Huang Y, Zhou Y, Yang W, et al.:
Identification and dissection of Ca2+-binding sites in the extracellular domain of Ca2+-sensing receptor.
J Biol Chem.
2007; 282(6): 19000–10. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 125.
Brown EM, Fuleihan G el-H, Chen CJ, et al.:
A comparison of the effects of divalent and trivalent cations on parathyroid hormone release, 3',5'-cyclic-adenosine monophosphate accumulation, and the levels of inositol phosphates in bovine parathyroid cells.
Endocrinology.
1990; 127(3): 1064–71. PubMed Abstract
| Publisher Full Text
- 126.
Ruat M, Snowman AM, Hester LD, et al.:
Cloned and expressed rat Ca2+-sensing receptor.
J Biol Chem.
1996; 271(11): 5972–5. PubMed Abstract
| Publisher Full Text
- 127.
Faurskov B, Bjerregaard HF:
Evidence for cadmium mobilization of intracellular calcium through a divalent cation receptor in renal distal epithelial A6 cells.
Pflugers Arch.
2002; 445(1): 40–50. PubMed Abstract
| Publisher Full Text
- 128.
Brown EM:
Is the calcium receptor a molecular target for the actions of strontium on bone?
Osteoporos Int.
2003; 14(Suppl 3): S25–34. PubMed Abstract
- 129.
Quinn SJ, Ye CP, Diaz R, et al.:
The Ca2+-sensing receptor: a target for polyamines.
Am J Physiol.
1997; 273(4 Pt 1): C1315–23. PubMed Abstract
- 130.
Katz CL, Butters RR, Chen CJ, et al.:
Structure-function relationships for the effects of various aminoglycoside antibiotics on dispersed bovine parathyroid cells.
Endocrinology.
1992; 131(2): 903–10. PubMed Abstract
| Publisher Full Text
- 131.
Ward DT, McLarnon SJ, Riccardi D:
Aminoglycosides increase intracellular calcium levels and ERK activity in proximal tubular OK cells expressing the extracellular calcium-sensing receptor.
J Am Soc Nephrol.
2002; 13(6): 1481–9. PubMed Abstract
| Publisher Full Text
- 132.
McLarnon S, Holden D, Ward D, et al.:
Aminoglycoside antibiotics induce pH-sensitive activation of the calcium-sensing receptor.
Biochem Biophys Res Commun.
2002; 297(1): 71–7. PubMed Abstract
| Publisher Full Text
- 133.
Brown EM, Katz C, Butters R, et al.:
Polyarginine, polylysine, and protamine mimic the effects of high extracellular calcium concentrations on dispersed bovine parathyroid cells.
J Bone Miner Res.
1991; 6(11): 1217–25. PubMed Abstract
| Publisher Full Text
- 134.
Ye C, Ho-Pao CL, Kanazirska M, et al.:
Amyloid-beta proteins activate Ca2+-permeable channels through calcium-sensing receptors.
J Neurosci Res.
1997; 47(5): 547–54. PubMed Abstract
| Publisher Full Text
- 135.
Stix B, Reiser G:
Beta-amyloid peptide 25–35 regulates basal and hormone-stimulated Ca2+ levels in cultured rat astrocytes.
Neurosci Lett.
1998; 243(1–3): 121–4. PubMed Abstract
| Publisher Full Text
- 136.
Conigrave AD, Quinn SJ, Brown EM:
L-amino acid sensing by the extracellular Ca2+-sensing receptor.
Proc Natl Acad Sci U S A.
2000; 97(9): 4814–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 137.
Conigrave AD, Mun HC, Brennan SC:
Physiological significance of L-amino acid sensing by extracellular Ca2+-sensing receptors.
Biochem Soc Trans.
2007; 35(Pt 5): 1195–8. PubMed Abstract
| Publisher Full Text
- 138.
Conigrave AD, Hampson DR:
Broad-spectrum amino acid-sensing class C G-protein coupled receptors: molecular mechanisms, physiological significance and options for drug development.
Pharmacol Ther.
2010; 127(3): 252–60. PubMed Abstract
| Publisher Full Text
- 139.
Wang M, Yao Y, Kuang D, et al.:
Activation of family C G-protein-coupled receptors by the tripeptide glutathione.
J Biol Chem.
2006; 281(13): 8864–70. PubMed Abstract
| Publisher Full Text
- 140.
Broadhead GK, Mun HC, Avlani VA, et al.:
Allosteric modulation of the calcium-sensing receptor by gamma-glutamyl peptides: inhibition of PTH secretion, suppression of intracellular cAMP levels, and a common mechanism of action with L-amino acids.
J Biol Chem.
2011; 286(11): 8786–97. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 141.
Nemeth EF:
Calcimimetic and calcilytic drugs: just for parathyroid cells?
Cell Calcium.
2004; 35(3): 283–9. PubMed Abstract
| Publisher Full Text
- 142.
Mayr B, Glaudo M, Schöfl C:
Activating Calcium-Sensing Receptor Mutations: Prospects for Future Treatment with Calcilytics.
Trends Endocrinol Metab.
2016; 27(9): 643–52. PubMed Abstract
| Publisher Full Text
- 143.
Lindberg JS, Culleton B, Wong G, et al.:
Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study.
J Am Soc Nephrol.
2005; 16(3): 800–7. PubMed Abstract
| Publisher Full Text
- 144.
Hebert SC:
Therapeutic use of calcimimetics.
Annu Rev Med.
2006; 57: 349–64. PubMed Abstract
| Publisher Full Text
- 145.
Brown EM:
Clinical utility of calcimimetics targeting the extracellular calcium-sensing receptor (CaSR).
Biochem Pharmacol.
2010; 80(3): 297–307. PubMed Abstract
| Publisher Full Text
- 146.
Henley C 3rd, Yang Y, Davis J, et al.:
Discovery of a calcimimetic with differential effects on parathyroid hormone and calcitonin secretion.
J Pharmacol Exp Ther.
2011; 337(3): 681–91. PubMed Abstract
| Publisher Full Text
- 147.
Ma JN, Owens M, Gustafsson M, et al.:
Characterization of highly efficacious allosteric agonists of the human calcium-sensing receptor.
J Pharmacol Exp Ther.
2011; 337(1): 275–84. PubMed Abstract
| Publisher Full Text
- 148.
Nemeth EF:
The search for calcium receptor antagonists (calcilytics).
J Mol Endocrinol.
2002; 29(1): 15–21. PubMed Abstract
| Publisher Full Text
- 149.
Balan G, Bauman J, Bhattacharya S, et al.:
The discovery of novel calcium sensing receptor negative allosteric modulators.
Bioorg Med Chem Lett.
2009; 19(12): 3328–32. PubMed Abstract
| Publisher Full Text
- 150.
Nemeth EF, Goodman WG:
Calcimimetic and Calcilytic Drugs: Feats, Flops, and Futures.
Calcif Tissue Int.
2016; 98(4): 341–58. PubMed Abstract
| Publisher Full Text

Comments on this article Comments (0)