Keywords
adrenomedullin 2 (ADM2), intermedin (IMD), protein kinase C (PKC), endothelial cells, signal transduction
adrenomedullin 2 (ADM2), intermedin (IMD), protein kinase C (PKC), endothelial cells, signal transduction
Adrenomedullin 2 (ADM2), also known as intermedin, is a secreted peptide that belongs to the calcitonin gene-related-peptide family1,2. It has been reported that ADM2 regulates intracellular calcium levels and contractile function in protein kinase C (PKC) - and protein kinase A (PKA) - dependent mechanisms in cardiomyocytes3. ADM2 activates the cAMP/PKA signaling pathway, which mediates inactivation of contractility and strengthening of cell-cell adhesion in endothelial cells4. ADM2 activates extracellular-signal-regulated kinase (ERK), a key signaling molecule for cell proliferation in endothelial cells5. To investigate whether ADM2 activates ERK through PKCα, which is a major upstream activator of ERK in endothelial cells we examined the effect of phosphorylation of ERK on ADM2 stimulation in endothelial cells isolated from PKCα null mice or wild type (wt) counterpart mice.
Animal care and experimental procedures were performed under protocol # CC0004 approved by the Institutional Animal Care and Use Committees of Yale University. Endothelial cells were isolated from wild type (C57BL/6J, The Jackson Laboratory, Cat # 000664) and PKCα-/- mice (Prkcatm1Jmk, The Jakson Laboratory, Cat # B6;129-Prkcatm1Jmk/J) and maintained as previously described6. Briefly, the arteries of both wild-type and knockout mice were harvested, finely minced with scissors, and digested with 25 ml collagenase (2 mg/ml) at 37°C for 45 min under gentle agitation. The crude preparation was triturated, passing it 12 times through a cannula needle, and was then filtered on a 70-μM sterile cell strainer. The filtered preparation was spun at 400 × g, and the pellet was resuspended in 2 ml of 0.1% BSA. Magnetic beads (Invitrogen) coated with anti-mouse CD31 (BD Biosciences) were added to the cell suspension and incubated with rotation at room temperature for 15 min. The bead-bound cells were recovered with a magnetic separator and washed with DMEM containing 20% FBS. Cells were suspended in 10 ml of complete DMEM and seeded on cell culture plates (Catalog # 353003, Corning Inc., Corning, NY). Subconfluent cells were serum-starved for 16h followed by incubation with 10 ng/ml ADM2 peptide (Pheonix Pharmaceuticals, Burlingame, CA) for the indicated time length: 0, 5, and 30min . Cells were lysed in RIPA buffer (Catalog # R0278, Sigma-Aldrich, St Louis, MI), supplemented with protease inhibitor cocktail (Catalog # 11 873 580 001, Roche Diagnostics, Mannheim, Germany) and phosphatase inhibitor cocktails (Catalog # P0044 and P5726, Sigma-Aldrich, St Louis, MI) as instructed by manufactures. Total cell lysates were subjected to immunoblotting analysis as described previously2. The membranes were hybridized with antibodies recognizing phospho-ERK (at 1:2,000 dilution of Catalog # 4370, Cell Signaling Technologies, Danvers, MA), total ERK (at 1:1,000 dilution of Catalog # 4695, Cell Signaling Technologies, Danvers, MA), PKCα (at 1:500 dilution of Catalog # 610108, BD BioSciences, San Jose, CA), and β-actin (at 1:10,000 dilution of Catalog # sc-47778, Santa Cruz Biotechnology Inc., Dallas, Texas). Following incubation with horseradish peroxidase-conjugated goat anti-rabbit or mouse IgG (Zymed Laboratories Inc., San Francisco, CA). Western signals were visualized with enhanced chemiluminescence (Thermo Fisher Scientific, Waltham, MA).
As shown in Figure 1, ADM2 increased phosphorylation of ERK in endothelial cells. However, there was no difference in ERK phosphorylation levels in wt versus PKCα null endothelial cells (Figure 1). Our results indicate that ADM2 activates ERK in endothelial cells via a PKCα – independent pathway.
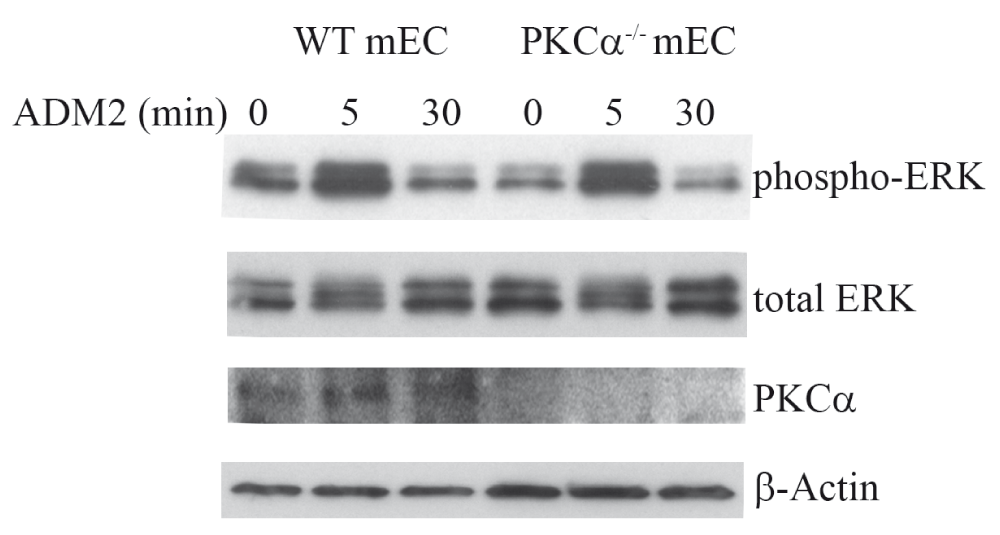
Representative immunoblot showing that ADM2 increased phosphorylation of ERK, via a PKCα-independent pathway, in endothelial cells. Mouse endothelial cells (mEC) were isolated from wild type (wt) and Protein kinase Cα knockout mice (PKCα-/-). Cells were serum-starved overnight followed by stimulation with ADM2 synthetic peptide (10ng/ml) for indicated time and cell lysates were analyzed by immunoblotting for ERK activation.
F1000Research: Dataset 1. Gel images for ‘Adrenomedullin 2 activates extracellular-signal-regulated kinase in endothelial cells via a protein kinase C α-independent pathway’ by Guo X., et al., 10.5256/f1000research.2420.d1106897
XG cultured and treated cells and performed Western analyses, and prepared the manuscript; RJ isolated endothelial cells from mice, CC and MS directed the study.
This work is supported by OHSE fund (Department of Surgery, Yale School of Medicine) funding to XG.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 06 Jan 16 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)