Keywords
Management, Mast Cell Activation Syndromes, Mastocytosis, Treatment
Management, Mast Cell Activation Syndromes, Mastocytosis, Treatment
Mast cells (MCs) are a key structural and functional component of the immune system and play a key role in inflammatory reactions, and at the same time they are the main effector cells in allergic processes1–3. MC disorders might present with a great variety of clinical symptoms or signs, such as skin involvement, which might lead to suspicion of the disease. Nevertheless, among other MC disorders, two entities frequently represent a diagnostic and therapeutic challenge in routine clinical practice in allergy: (i) indolent systemic mastocytosis presenting without skin involvement (ISMs−) and (ii) clonal mast cell activation syndrome (c-MCAS). Of note, both entities are closely related to anaphylaxis, and their diagnosis requires specific techniques. Here, we review the current consensus and guidelines for the diagnosis, classification, treatment, and management of these two entities.
The term systemic mastocytosis (SM) is used to define a heterogeneous group of rare diseases characterized by the presence of abnormal MCs in various organs and tissues4. Two critical biological findings which are linked to the pathogenesis of the disease have been described: (i) activating somatic mutations in the KIT gene (usually the KIT Asp816Val D816V mutation) and the presence of an aberrant immunophenotype associated with the expression of CD25 on (bone marrow [BM]) clonal MCs. The current World Health Organization (WHO) classification of the disease includes up to seven distinct categories that meet the diagnostic criteria for mastocytosis (Table 1). However, the development of new, more sensitive and specific methods, such as multi-parameter flow cytometry and highly sensitive polymerase chain reaction (PCR)-based techniques for the detection of aberrant MCs present at very low frequencies5–8 and the study of the KIT mutation in purified cells9 or blood10–12 or both, have led to an unprecedentedly increased rate of detection of phenotypically aberrant and KIT mutated MCs in BM and peripheral blood, pointing out not only the potential need to revise current diagnostic and classification criteria to recognize new entities with very low tumor burden associated with life-threatening symptoms such as anaphylaxis but also a potential impact on the long-term prognosis of patients with indolent forms of the disease.
| Classification of mastocytosis | Diagnosis |
|---|---|
| Cutaneous mastocytosis - Maculopapular cutaneous mastocytosisa - Diffuse cutaneous mastocytosis - Mastocytoma of skin | - >15 mast cells (MCs) aggregating or more than 20 MCs per high- power field microscopy ×(40) in skin biopsy - Absence of systemic mastocytosis (SM) criteria |
| SM - Indolent SM (with or without skin involvement) - Smoldering SM - Aggressive SM - SM with an associated hematological neoplasm - MC leukemia | - SM criteria - Absence of C-findingsb and other clonal hematological diseases -<20% of MCs in bone marrow (BM) sections - SM criteria - Absence of C-findingsb and other clonal hematological diseases but two or more B-findingsb - SM criteria - C-findingsb - SM criteria - Demonstration of a clonal hematological non-MC disease - SM criteria - >20% of MCs in BM sections |
| MC sarcoma | - Infiltration of an extracutaneous organ by undifferentiated MCs with a destructive growth pattern |
aFormerly known as urticaria pigmentosa. Main type of cutaneous mastocytosis82.
bB-findings include (i) infiltration grade (MC) in BM of more than 30% and serum tryptase of more than 200 ng/mL, (ii) dysmyelopoiesis, and (iii) organomegaly without impariment of organ function4. C-findings indicate organ dysfunction due to widespread MC infiltration, including cytopenias, osteolysis, malabsorption, and organomegaly with functional impairment of the organ/tissue (hypersplenism, portal hypertension, ascites)4.
Diagnostic criteria: At least one major criterion and one minor criterion or at least three minor criteria must be fulfilled for the diagnosis of SM to established. Major diagnostic criteria: multifocal dense infiltrates of MCs (>15 MCs aggregating) detected in BM sections and/or other extracutaneous organ(s) by tryptase immunohistochemistry or other MC-associated stains. Minor diagnostic criteria: (1) more than 25% of MCs are spindle-shaped in MC infiltrates detected in BM sections or other extracutaneous tissue sections OR of more than 25% atypical MCs (type I plus type II) detected in BM smears; (2) detection of a KIT point mutation at codon 816 in BM MCs or other extracutaneous organ(s); (3) expression of CD25 or CD2 (or both) on MCs in BM MCs, blood, or other extracutaneous tissues; (4) total serum baseline tryptase concentration persistently more than 20 ng/mL (in case of an associated hematologic non-MC lineage disease, this criterion is not valid).
Based on previous reports in the largest series of patients, indolent systemic mastocytosis (ISM) comprises around 80% of all SM cases13. Among them, around 20% of patients lack skin lesions at presentation (ISMs−)14. Despite the great relevance and efficiency of the WHO criteria for the diagnosis of SM, in ISMs−, MCs represent only a very small proportion of all nucleated BM cells (usually fewer than 10−3 BM MCs, as assessed by flow cytometry)15, and BM MC aggregates are frequently (around 30% of cases) not found in such patients with SM15, in the absence of significantly increased serum baseline tryptase levels (<20 μg/L). Consequently, the use of highly sensitive and specific methodological approaches to the study of BM MCs becomes critical in order to avoid a misdiagnosis in patients presenting with low tumor burden16.
The term MC activation syndrome (MCAS) encompasses a heterogeneous group of diseases which are characterized by systemic symptoms secondary to MC mediator release that (i) might or might not have a known trigger, (ii) might or might not be associated with immunoglobulin E (IgE)-specific antibodies in response to that trigger, (iii) are associated with normal or elevated baseline tryptase levels, and (iv) do not show skin lesions of mastocytosis17. In Table 2, the most frequent and relevant clinical symptoms suggesting an underlying MCAS are listed, and Table 3 depicts the diagnostic criteria for MCAS.
LT, leukotriene. Adapted with permission from Karger17.
| Criteria |
|---|
| 1. Typical clinical symptomsa |
| 2. Increase in serum total tryptase by at least 20% above baseline plus 2 ng/mL during or within 4 hours after a symptomatic period |
| 3. Response of clinical symptoms to histamine receptorb blockers or “mast cell- targeting” agents (for example, cromolyn) |
aDifferent clinical symptoms are suggestive of systemic mast cell activation syndrome (MCAS). The following reached a consensus level above 70%17: flushing, pruritus, urticaria, angioedema, nasal congestion, nasal pruritus, wheezing, throat swelling, headache, hypotension, and diarrhea. None of them per se is specific for MCAS and thus can count as MCAS criteria only in the context of the other two criteria.
bHistamine receptor blockers: H1 ± H2 inverse agonists
Reproduced with permission from Karger17
The current classification of MCAS is shown in Table 4. Based on the experience of the Spanish Network of Mastocytosis (REMA), the most relevant objective criteria to subclassify MCAS rely on the presence versus absence of clonal MCs as defined by the expression of CD25 (for example, CD25+ versus CD25−) or a KIT mutation, particularly KIT D816V, or both. When MCAS diagnostic criteria are fulfilled but there is no evidence of clonality, non-clonal-MCAS should be considered and co-existence of allergy or other underlying diseases should be confirmed or ruled out18.
| Diagnostic categories and variants | Proposed criteria |
|---|---|
| Primary mast cell activation syndrome (MCAS) | MCAS and clonality criteria are met (CD25+ or KIT D816V mutated MCs or both)a |
| Mastocytosis | |
| Clonal or monoclonal MCAS (c-MCAS) | |
| Secondary MCAS | MCAS, allergy, or other mast cell (MC)- activating diseases criteria are met |
| Allergy | |
| Other underlying diseasesb | |
| Idiopathicc MCAS | MCAS criteria are met but the diagnosis of the disease that explains MC activation is not achieved |
aCD25+ KIT D816V mutated MC or KIT D816V mutated MCs without CD25+ expression
bIncludes autoimmune diseases, bacterial infections, and drug adverse reactions
cThis is an exclusion diagnosis and therefore a complete study is needed in order to discard any known disease that might cause MC activation
Reproduced with permission from Karger17
Symptoms due to the release of MC mediators upon MC activation might be present in every category of MCAS, including mild, severe, or even life-threatening symptoms such as pruritus, flushing, gastrointestinal complaints (abdominal pain or diarrhea), cognitive symptoms, and even anaphylaxis19,20.
In indolent systemic mastocytosis with skin lesions (ISMs+), MC activation symptoms are typically heterogeneous and might vary from recurrent anaphylaxis21,22 to occasional symptoms triggered by a varying number of different stimuli linked to the MC mediator release episodes (Table 5). In turn, ISMs− are frequently characterized by serious episodes of MC mediator release triggered by different factors—for example, mainly insect sting, drugs, and foods—or they might be idiopathic23; in both situations, such episodes are significantly associated with the presence of anaphylaxis with cardiovascular or vascular collapse symptoms, in the absence of both urticaria and angioedema23. Early studies by the REMA have demonstrated that ISMs− patients present unique features that distinguish them from ISMs+ cases23: (i) a higher prevalence of men versus women, (ii) a lower frequency of symptoms outside of acute episodes, (iii) lower BM MC burden, and (iv) the presence of the KIT mutation usually restricted to the MC lineage23,24. Of note, such unique disease features are even more characteristic within ISMs− patients whose symptoms are triggered exclusively by insect stings, whereas ISMs− patients with other triggering factors show clinical characteristics at presentation which are more similar to those of ISMs+ cases24.
| Trigger | Recommendations |
|---|---|
| Physical agents | |
| - Heat, changes in temperature | - Use air conditioning when necessary and mildly warm water for bath/ shower |
| - Friction on mastocytomas | - Avoid Darier’s sign |
| - Manipulation of the GI system (for example, during surgery) | - Consider prophylactic anti-mediator therapy |
| Emotional factors | |
| - Stress, anxiety | - Consider anxiolytics or relaxation techniques or both |
| Drugs | |
| - NSAIDsa | - Use drugs with known tolerance for each case and consider drug challenge testing whenever tolerance is unknown and the drug required |
| - Opioids | - Use drugs with known tolerance for each case and consider drug challenge testing whenever tolerance is unknown and the drug required |
| - Anestheticsb,c | - Use drugs with known tolerance for each case and consider prophylactic anti-mediator therapy and anesthetic drugs with the safest profile |
| - Radiological contrast mediac | - Use contrast media with known tolerance for each case and consider prophylactic anti-mediator therapy and use low-molecular-weight contrast agents |
| - Interferon α2b | - Consider prophylactic anti-mediator therapy before first doses |
| - Cladribined | - Consider prophylactic anti-mediator therapy before first doses |
| - Vaccinesc,e | - Consider prophylactic anti-mediator therapy |
| - Dextrans | - Use low-molecular-weight dextran or alternative solutions |
| Insect sting and bites | |
| - Hymenoptera | - Use insect repellents; avoid perfumed lotions; wear light-colored clothes, long-sleeved shirts, and long pants; avoid going barefoot; patients with history of previous insect-induced anaphylaxis must carry an emergency kit; specific immunotherapy for IgE-mediated hymenoptera venom allergy is recommended |
| - Hippobosca equina, mosquitof | - Use insect repellents; avoid perfumed lotions; wear light-colored clothes, long-sleeved shirts, and long pants; avoid going barefoot; patients with history of previous insect-induced anaphylaxis must carry an emergency kit |
aFrequency of mast cell (MC) mediator-related symptoms of 2% in pediatric mastocytosis and 14% in adult mastocytosis83
bFrequency of MC mediator-related symptoms and anaphylaxis of 2% and 0.4% in adult mastocytosis, respectively84; 4% of MC mediator-related symptoms and 0–2% of anaphylaxis in pediatric mastocytosis84–86
cProphylactic anti-mediator therapy is recommended in all cases87
dInfrequent, based on one case report (Javed Sheik, Beth Israel Hospital, Harvard Medical School, personal communication, September 2002)
eInfrequent, based on case report88
fInfrequent, based on case reports89,90
GI, gastrointestinal; IgE, immunoglobulin E; NSAID, non-steroidal anti-inflammatory drug. Adapted with permission from Ergon91.
The final diagnosis of SM and c-MCAS systematically requires a BM study for the evaluation of all disease characteristics used for the diagnosis of SM, such as BM MC cytology25, histology and immunochemistry26,27, flow cytometry immunophenotyping using specific gating strategies for the detection of BM MCs present at low frequencies5–7,28, and the study of KIT mutation in purified MCs9,29, together with a detailed clinical work-up. Usually, these studies are available only in reference centers, and therefore either the patient or the samples should be referred.
The European Competence Network on Mastocytosis recommends using the REMA score (Figure 1) as a clinically useful tool to predict for the presence of clonal MCs prior to a BM study23; the REMA score is based only on demographic data (gender), the symptoms and signs observed during the acute episodes, and serum baseline tryptase levels. A REMA score of at least 2 predicts with a high sensitivity and specificity for ISMs− (or c-MCAS), whereas a REMA score of less than 2 usually indicates non-clonal disease. Whether “non-clonal disease” means a true absence of any mutations whatsoever or simply the absence of clonality currently detectable in the clinical laboratory remains unclear. The REMA score is a particularly helpful tool since (i) it is based on clinical data and can be used on a routine clinical basis, (ii) it is associated with rather low costs, and (iii) it avoids unnecessary BM studies.
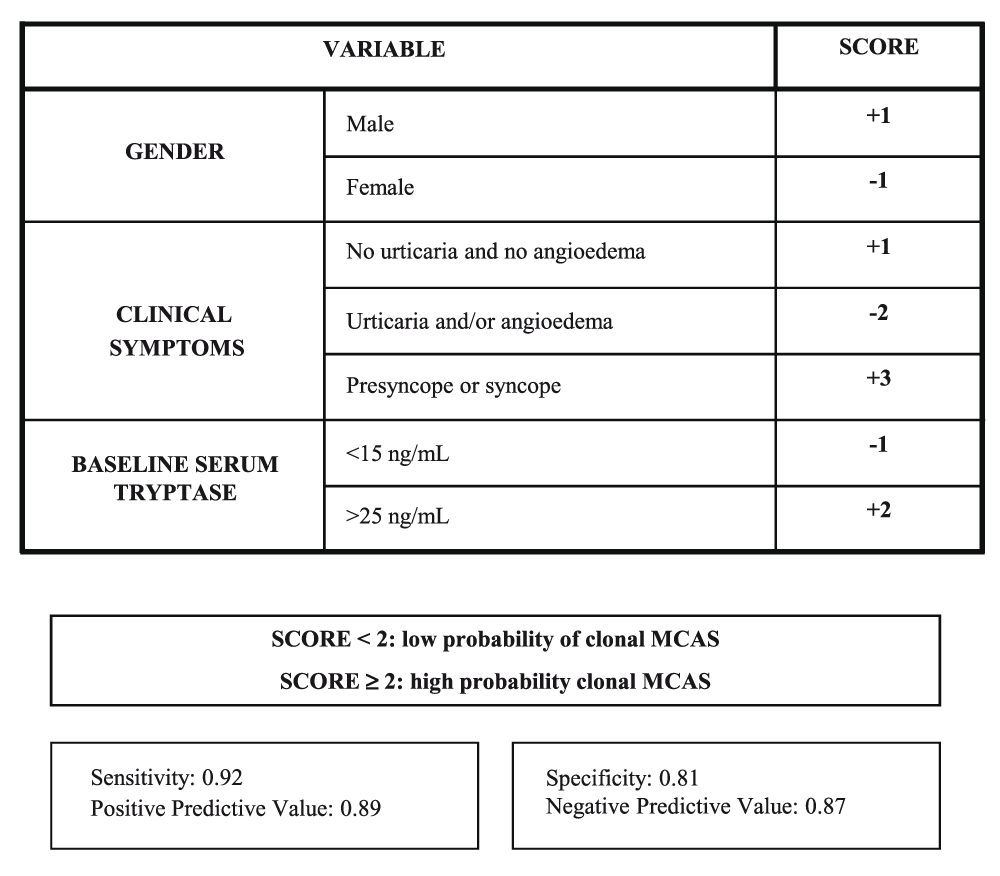
This scoring model is proposed as a screening tool for the diagnosis of clonal mast cells in patients presenting with anaphylaxis in the absence of skin mastocytosis before a bone marrow study is performed. MCAS, mast cell activation syndrome. Reproduced with permission from Elsevier Inc.23.
Whenever a patient is suspected of having a primary MCAS (either mastocytosis without skin involvement or c-MCAS), the use of the REMA score mentioned above is recommended23 as an initial screening measure (Figure 2). Patients with a REMA score of less than 2 will have a low probability of presenting clonal MCs; therefore, in such cases, it is usually not necessary to conduct additional studies other than controlling the symptoms with adequate medication and scheduling periodic follow-up visits. In contrast, patients with a REMA score of at least 2 have a high probability of presenting mastocytosis or c-MCAS. Thus, in the latter patients, it is recommended that appropriate treatment be started and that the patient be evaluated in detail for major complications of the disease, such as hepatomegaly/splenomegaly, osteopenia, and osteoporosis. To establish the most appropriate timing to perform a BM biopsy to arrive at a firm diagnosis, it might be useful to evaluate the presence of the D816V KIT mutation in peripheral blood (it is typically positive in approximately 80% of primary MCAS cases12) and to monitor baseline serum tryptase levels and wait until they rise above 20 ng/mL, at which point the probability of obtaining a BM sample that is suitable for demonstrating BM involvement increases. Independently of the time at which the BM study is scheduled, it is recommended that BM MCs be purified prior to molecular studies, which have demonstrated greater sensitivity for the detection of KIT mutations9,23,30. As an exception, BM biopsy studies might be performed in suspicious patients with a REMA score of at least 2 and baseline tryptase levels of less than 20 ng/mL who presented with anaphylaxis following a hymenoptera sting and who are candidates for immunotherapy, since patients with mastocytosis or c-MCAS (or both) have a greater risk of having adverse reactions during administration of venom immunotherapy (VIT)14 (see the ‘Hymenoptera venom immunotherapy’ section below). A new quantitative real-time PCR test for KIT-D816V that is substantially more sensitive than standard KIT-D816V PCR testing has recently been developed, as the quantitative reverse transcription-PCR (qrt-PCR) test is showing essentially 100% sensitivity31. Unfortunately, the availability of the qrt-PCR test at present is far more limited than that of the standard PCR test.
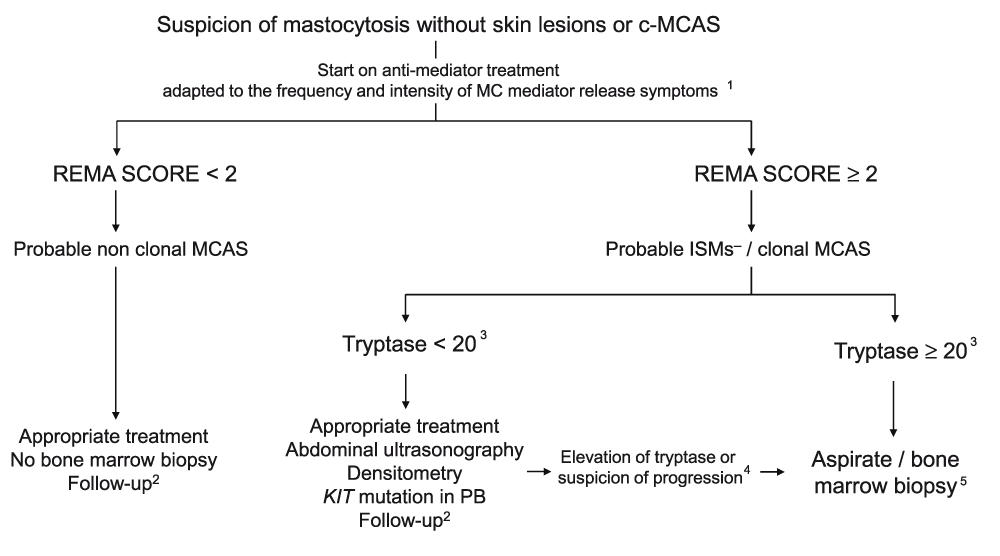
Clonality in this figure (as in Figure 1) is limited to positivity for KIT-D816V by polymerase chain reaction or positivity for co-expression by flow cytometry of CD117 with CD25 or CD2 or both.
1In asymptomatic patients, only sodium cromoglicate. Depending on additional symptoms, assess adding other anti-mediator treatments.
2Periodic determination of tryptase together with follow-up of clinical evolution and, if necessary, image tests.
3Tryptase values are approximate and are based on the fact that, in patients with low values, the percentage of MCs in bone marrow is very low and therefore the possibility of finding aggregates or identifying MCs is more complicated.
4In cases where there is a rising trend in baseline tryptase values, clinicians are advised to wait until it rises above 20 ng/mL, at which point the probability of obtaining a sample that is suitable for conducting the study increases. The unique situation in which a bone marrow biopsy can be assessed in patients with a score of at least 2 and baseline tryptase levels of less than 20 ng/mL occurs when the patient has presented anaphylaxis following a hymenoptera sting and is a candidate for immunotherapy, given that patients with mastocytosis or c-MCAS (or both) have a greater risk of having adverse reactions during the administration of venom immunotherapy.
5The bone marrow study should be done only if the mentioned methods (see text, section “Diagnosis of primary mast cell activation syndromes”), and flow cytometry and cell purification in particular, are available. If the technology needed to conduct these studies is not available, it is recommended that patients be referred to specialized reference centers.
c-MCAS, clonal mast cell activation syndrome; ISM−, indolent systemic mastocytosis without skin lesions; MC, mast cell; MCAS, mast cell activation syndrome; PB, peripheral blood; REMA, Spanish Network on Mastocytosis. Adapted with permission from Ergon91.
Patients with c-MCAS or mastocytosis may present symptoms due to the release of MC mediators, associated or not with symptoms related to tissue infiltration by clonal MC (more frequent among aggressive forms of mastocytosis). At present, there is no curative therapy for mastocytosis and the treatment strategies are focused on controlling the frequency and intensity of symptoms secondary to the release of MC mediators; this includes both strict avoidance of triggers (Table 5) and the anti-mediator therapy carefully selected on the basis of the intensity or severity (or both) of the signs and symptoms linked with the activation of MCs. Cytoreductive therapy and targeted therapies with tyrosine kinase inhibitors might be needed in selected cases presenting with elevated MC burden or aggressive and poor prognosis-associated forms of SM or patients who are unresponsive to conventional anti-mediator therapy32, but these therapies will not be discussed further in this review.
Careful counseling must be given to patients, caretakers, and their physicians to avoid triggers that evoke MC mediator release32,33 (Table 5). In addition, detailed training to manage MC activation-associated episodes should be provided to patients as well as their physicians.
Anti-mediator therapy for primary MCAS aims at inhibiting production, interfering with release, blocking the specific receptors, or antagonizing the effects of MC mediators. It is used both to treat and to prevent acute and chronic MC mediator release-associated symptoms32. The specific anti-mediator administration schedule (on demand or continuous administration) should be carefully selected for the individual patient on the basis of the intensity or severity (or both) of the signs and symptoms observed during the most severe acute episodes or anaphylaxis as well as the MC mediator-related symptoms recorded between acute episodes19.
Histamine receptor blockers. The biologic effects of histamine released from MCs, through its binding to histamine receptors (mainly H1 and H2 histamine receptors), include (i) increased vascular permeability, (ii) vasodilatation, (iii) contraction of non-vascular smooth muscle, (iv) increase of exocrine gland secretion, and (v) stimulation of the peripheral nervous system; these effects result in symptoms such as pruritus, urticaria, edema, bronchoconstriction, gastric hypersecretion, abdominal cramping, diarrhea, headache, hypotension, and anaphylaxis34. The effects of H1 blockers are described in Table 6.
| Drug | Mechanism of action | Controlled symptoms |
|---|---|---|
| H1 antihistamines | Histamine receptor blocker | Pruritus, flushing, urticaria, swelling, tachycardia, abdominal pain related with MC degranulation processes, hypotension or reduction of the severity of symptoms of anaphylaxis32,34,42,92,93 |
| H2 antihistamines | - Histamine receptor blocker that can potentiate the effect of H1 antihistamines | Gastric hypersecretion, abdominal pain, diarrhea, and recurrent/severe MC mediator release episodes32,40,42,94,95 |
| Sodium cromol | - Unclear - Inhibits GTP-g-S-induced exocytosis in MCs and modulates sensory nerve function106,107 | Abdominal pain, vomiting, diarrhea (based on double- blind placebo-controlled trials), pruritus, flushing, headache, cognitive and skeletal symptoms40,45,96–105 |
| Aspirin and NSAIDs | Inhibition of cyclooxygenase and blockade of the synthesis of PGD2 | Flushing, dizziness, and gastrointestinal symptoms48,108 |
| Montelukast | Antagonizes cysLT receptor 1 | Respiratory, cutaneous, gastrointestinal, and urinary symptoms47,109–111 |
| Zileuton | Blockade of the synthesis of LTs by inhibiting LO | Neuropsychiatric and constitutional subjective symptoms |
| Glucocorticoids | - Binding the intracellular glucocorticoid receptor and modulation of the transcription mediator of numerous genes - Decreased number of connective tissue MCs - Inhibition of the production of stem cell factor production and other interleukins and eicosanoid mediators - Decreased expression of chemokine receptors (for example, CCR3) - Decreased MC activation via induction of decreased expression of FcεRI - Inhibition of signaling cascades in MCs through the expression of phosphatases that are upregulated by glucocorticoids42,114 | Gastrointestinal malabsorption, abdominal pain, ascites, bone disease (including diffuse bone sclerosis); acute and/or severe MC release40,112,113 |
| Omalizumab | Blocks the binding of IgE to the FcεRI receptor on the surface of MCs and basophils, reducing receptor expression123 and the release of mediators124 | Cutaneous symptoms115–117, gastrointestinal symptoms118,119, anaphylaxis118,120–122, and reactions to venom immunotherapy58 |
Some H1 antihistamines such as desloratadine and ketotifen have MC-stabilizing properties, and therefore they might also decrease the release of MC mediators35,36. Ketotifen has been reported to be effective in the treatment of bone disease as well as cutaneous, gastrointestinal, and neuropsychiatric symptoms in mastocytosis37–39. Patients with associated depression might benefit from doxepin because of its effects as an antidepressant and H1 histamine blocker40. Rupatadine also has an antagonistic effect against platelet-activating factor, a lipid-derived mediator which is newly synthesized and released by MCs upon their activation, resulting in hypotensive episodes and flushing41.
Some patients with mastocytosis require a combination of different H1 blockers to achieve a good control of symptoms32. The use of non-sedating H1 blockers is recommended for patients who require daily maintenance therapy; in turn, the use of sedating H1 antihistamines that have a fast-acting effect which makes them suitable drugs to treat acute MC mediator release episodes, frequently in association with corticosteroids or epinephrine or both, and also to prevent MC degranulation during risk situations might be administered at night or on demand32,42.
Cromolyn sodium. Cromolyn sodium is an MC stabilizer that has been proven to inhibit MC activation and MC release of mediators both in vitro and in vivo despite its limited systemic absorption following ingestion; this suggests an inability of the drug to enter cells and the need for a potential interaction with an as-yet-unidentified cell surface receptor to induce its biologic activity43 (Table 6). Side effects include headache, sleepiness, irritability, abdominal pain, diarrhea, and constipation, most of which are attenuated by progressive introduction of the drug44. In addition, 0.21–4% cromolyn sodium water-soluble creams, as well as aqueous-based skin lotion, may be effective at improving cutaneous symptoms (for example, pruritus and flaring of lesions)32,34,45,46.
Antagonists of arachidonic acid metabolites. MCs synthesize de novo mediators and release arachidonic acid metabolites through the action of lipoxygenase and cyclooxygenase enzymes; thus, they produce leukotrienes and prostaglandins (PGs), respectively. Prostaglandin D2 is generated almost entirely by MCs and rapidly converted into active metabolites of prolonged activity rather than the parent compound, such as α11β-PGF2; the two PGs share a biological activity and induce bronchoconstriction, increase of vascular permeability, and vasodilatation, and at the same time they have chemoattractive properties for eosinophils, basophils, and Th2 lymphocytes47 and are involved in the development of flushing and possibly also hypotensive episodes in patients with c-MCAS and mastocytosis48 (Table 6).
Aspirin. Non-steroidal anti-inflammatory drugs, especially aspirin, can also inhibit the activation of MCs and their degranulation in some patients with mastocytosis; therefore, this therapy is recommended in patients with known tolerance to these drugs.
Glucocorticoids. The mechanism of action and the effects of glucocorticoids are shown in Table 6. Short cycles of low doses of either prednisone (0.3 mg/kg per day) or oral budesonide (0.1 mg/kg per day) may improve abdominal pain refractory to treatment with cromolyn. Topical corticosteroids may be used for patients who present with skin symptoms, especially among cases with limited skin involvement; however, evidence supporting their topical use is either anecdotal or based on small series of patients49.
Anti-immunoglobulin E therapy. Successful anti-IgE therapy has been documented in some conditions such as severe persistent allergic asthma, chronic urticaria50, idiopathic anaphylaxis51, and mastocytosis (Table 6). Until more information based on clinical trials becomes available, omalizumab in MC diseases should be restricted to selected patients with severe symptoms which have proven unresponsive to intensive anti-mediator therapy33.
Hymenoptera venom immunotherapy. Specific VIT is recommended for mastocytosis and c-MCAS patients with IgE-mediated anaphylaxis to hymenoptera venom; however, it should be managed as a high-risk procedure. VIT has proven effective and safe in these patients52–56; it has a rate of protection from re-stings of 86%, and the frequency of systemic reactions to VIT ranges from 5–25%14,57, most of which (75%) were associated with rush inductions (versus 25% using conventional induction)57. Whenever adverse reactions to VIT prevent the protective maintenance dose of 100 μg per month from being reached, prophylactic anti-mediator therapy, changes in the extract, and administration of omalizumab therapy may be useful57,58. Furthermore, in patients who present anaphylaxis after re-sting, despite the administration of a standard maintenance dose, it is recommended that maintenance doses be increased to 200 μg57. An extended maintenance administration (even lifelong) is proposed14,57 since cases presenting with fatal reactions after discontinuation of VIT have been described59,60.
Ultraviolet irradiation. In vitro studies demonstrated that long-wave ultraviolet radiation (psoralen plus ultraviolet A or ultraviolet A alone) and narrowband ultraviolet B phototherapy irradiation interfere with the release of histamine from skin-activated MCs and induce MC apoptosis61–63; all of them were employed as a second-line therapy to treat cutaneous symptoms (pruritus, whealing, and flare reactions) in patients with typical mastocytosis skin lesions who were not responsive to first-line therapies with anti-mediator drugs40,64–66. There is no information regarding whether these therapies are useful in ISMs− and c-MCAS cases. In addition, fading of hyperpigmented skin lesions (frequently temporary) can be observed in some cases. Furthermore, responses of life-threatening MC mediator release episodes in children with bullous diffuse cutaneous mastocytosis have been reported67.
Treatment of bone mass loss. This is a frequent finding in both ISMs+ and advanced mastocytosis but is less frequently observed among primary MCAS cases, particularly ISMs− and c-MCAS. Fractures are usually developed as a consequence of severe osteoporosis or large osteolytic lesions. Local MC infiltration and disturbances in bone remodeling which are due to the release of MC mediators such as interleukin-6 (IL-6), histamine, heparin, receptor activator of nuclear factor kappa-B ligand, osteoprotegerin, or sclerostin are involved in the pathogenesis of bone manifestations in mastocytosis68,69. Calcium and vitamin D supplements, combined with bisphosphonates, are usually the first choice for osteopenia and osteoporosis, respectively70–72. Other therapies such as estrogen replacement in postmenopausal women and denosumab or interferon-alpha73,74 in patients with severe osteoporosis at risk of pathologic bone fractures unresponsive to conventional treatments might also be considered. Regarding peptidergic/peptidomimetic drugs such as teriparatide, caution is urged since these drugs have been associated with driving MC activation via MRGPRX275.
Selective serotonin reuptake inhibitors. MCs contain numerous mediators, including neurotransmitters (for example, serotonin), cytokines, and chemokines, that play a role in stress response, behavior, and emotion regulation76, 77. Furthermore, the elevation of the circulating levels of tumor necrosis factor-alpha and IL-6 with, for example, endotoxin leads to depressive symptoms, and it has been previously described that some selective serotonin reuptake inhibitors (SSRIs) such as citalopram can reduce endotoxin-induced symptoms78. Based on this information, together with their good tolerability profile, SSRIs have emerged as an option to improve symptoms of depression in patients with mastocytosis79.
With regard to pregnancy, two different series of women with mastocytosis have been reported in the literature80,81. The larger series81 included 45 pregnancies and deliveries in women with non-aggressive categories of the disease. Based on their results, anti-mediator therapy (dexchlorpheniramine, loratadine, cetirizine, ranitidine, oral corticosteroids, and adrenaline), if required during pregnancy, as well as systematic administration of prophylactic anti-mediator therapy at the beginning of labor based on drugs with a good well-known safety profile, is recommended81.
BM, bone marrow; c-MCAS, clonal mast cell activation syndrome; IgE, immunoglobulin E; IL, interleukin; ISM, indolent systemic mastocytosis; MC, mast cell; MCAS, mast cell activation syndrome; PCR, polymerase chain reaction; PG, prostaglandin; qrt-PCR, quantitative reverse transcription-polymerase chain reaction; REMA, Spanish Network on Mastocytosis; SM, systemic mastocytosis; SSRI, selective serotonin reuptake inhibitor; VIT, venom immunotherapy; WHO, World Health Organization.
This work was supported by grants from the Fondo de Investigaciones Sanitarias of the Ministerio de Sanidad y Consumo of Spain, Fundación Mutua Madrileña, and Sociedad Española Alergia e Inmunología Clínica 2014 and 2015 (Spain).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 14 Nov 16 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)