Keywords
Malaria Box, Plasmodium falciparum
Malaria Box, Plasmodium falciparum
Malaria killed about half a million people in the year 2015, and 70% were children under the age of five1. The emergence and spread of resistance towards frontline anti-malarial drugs in South-East Asia has created an urgent need to discover new drugs. In addition, new drugs are needed to meet the objective of malaria elimination and global eradication, for which the currently available drugs are not adequate2. The desirable characteristics of new clinical candidates, also called Target Compound Profile (TCP), include high potency and fast killing of the asexual erythrocytic stage for quick relief of symptoms, high plasma half-life to reduce treatment duration, activity against the sexual stages to prevent transmission, activity against the liver-stage to avoid relapse and for prophylactic use, activity against multiple species of Plasmodium, and reduced propensity for the development of resistance3. New anti-malarial drugs must also be safe for mass administration, and for children and pregnant women, who are most vulnerable to malaria3.
It is currently not well understood how TCP properties are related to each other. This makes it difficult to assess whether it would be feasible for a single compound to have all TCP properties and what strategies could be adopted to find such candidates. With the discovery of thousands of active compounds from the high-throughput assays against the erythrocytic stage of P. falciparum4–8, it has become imperative to find a prioritization strategy that can identify the most promising candidates for further development. For a subset of antimalarial compounds identified from high-throughput screens in the so-called “Malaria Box”, many of the TCP properties have been assessed. The Malaria Box is a set of 400 compounds selected based on their potent activity against the erythrocytic stage of P. falciparum, chemical diversity and commercial availability9. These compounds were made available free of cost to researchers, thus catalysing a number of studies, including the screening of these compounds against multiple Plasmodium stages, eukaryotic pathogens and human cells10. Some of these compounds have also been tested for their propensity for resistance generation11. Here, we utilized the large amount of data generated on Malaria Box compounds and found significant associations between different TCP properties. Based on these observations, we propose a prioritization strategy for anti-malarial compounds for further development.
The screening data on Malaria Box compounds was obtained from Van Voorhis et al,10 who compiled the previously published data on Malaria Box compounds (55 assays), as well as their own data (236 assays). We rank transformed all assay values, such that higher values represent higher inhibition.
In case multiple assays were available for a given stage or concentration, their median values were taken: there were nine assays reporting EC50 values against the asexual stage of P. falciparum, one assay against asexual stage at high compound concentration (10 µM), five gametocytocidal assays conducted at 0.5–1 µM compound concentrations, ten gametocytocidal assays conducted at 2.5–5 µM compound concentrations and six gametocytocidal assays conducted at 10–12.5 µM compound concentrations. There was one assay each at lower and higher compound concentrations against liver (5 µM and 50 µM, respectively) and ookinete stages (1 µM and 10 µM, respectively). Values were also similarly combined for parasites with multiple assays, such as Babesia sp. and Mycobacterium tuberculosis.
The data on the in vitro resistance evolution of Malaria Box compounds was obtained from Corey et al.11.
All statistical analyses were performed in the R software v3.3.1 (https://www.r-project.org/). R commands hclust and prcomp were used for hierarchical clustering and PCA analyses respectively. PCA analyses were performed on the activity data against different Plasmodium stages and human cells. Rank correlation values were used to create the distance matrix for the hierarchical clustering.
Van Voorhis et al. have recently reported their large-scale screening data on Malaria Box compounds, which was compiled along with the previously published data10. We first reduced the dimensionality of this data by combining variables that describe activity against the same Plasmodium stage and pathogen. Assays conducted at higher concentration of compounds may provide different results from those performed at lower concentration, and thus assays conducted at different concentrations were combined separately.
Figure 1 provides an overview of the relationship between different properties of Malaria Box compounds in the form of a correlation matrix. Multiple observations can be made from this matrix. There is a moderate, but significant correlation, between activity against the asexual stage and the gametocyte stage of P. falciparum (Spearman rank correlation 0.43, between EC50 values and % inhibition of gametocytes at 1 µM compound concentration). The correlation between EC50 values against asexual stage and gametocyte stage gets lower when gametocytocidal activity was screened at higher compound concentrations (Spearman rank correlation 0.17, at 10µM). The gametocytocidal activity at higher concentrations shows a higher correlation with inhibitory activity against different pathogens, including M. tuberculosis and human fibroblast cells (Figure 1). Assays conducted at higher concentrations against asexual, liver and ookinete stages also show a higher correlation with toxicity against human cells and other cell types (Figure 1). These observations suggest that assays conducted at high compound concentration show general toxicity against a wide variety of cells, including human cells, thus hits identified from these assays should be used with caution.
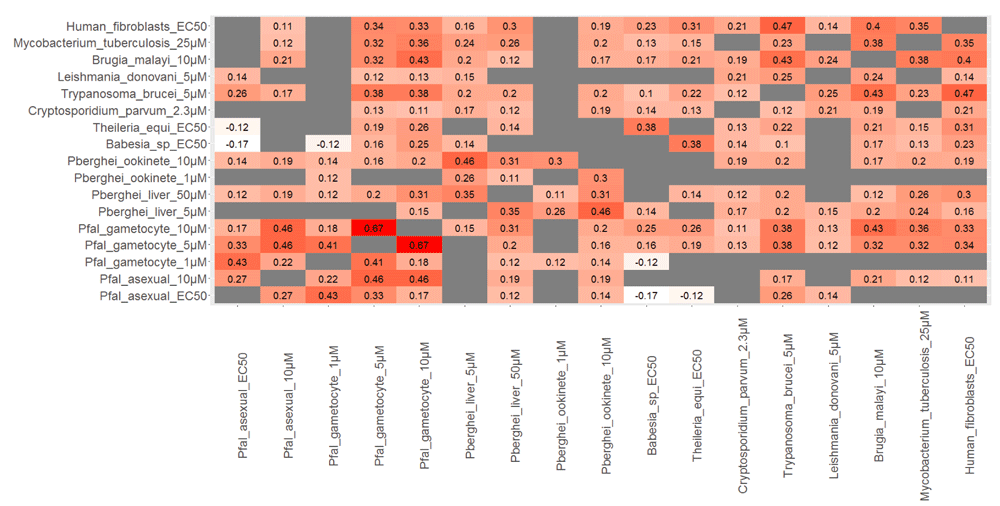
Spearman rank correlations are shown between assays whose values were rank transformed, such that higher values indicate higher inhibition. Gray boxes indicate p values > 0.05. The assays performed at higher concentrations in Plasmodium show higher positive correlations across different assays, including activity against human cells, suggesting that assays conducted at high compound concentration show general toxicity.
To further understand the relationship between activities against different Plasmodium stages, we performed hierarchical clustering of the data. Three major clusters were evident (Figure 2). Cluster 1 consists of asexual and gametocyte assays conducted at high compound concentrations. Cluster 2 consists of asexual and gametocyte assays conducted at low compound concentrations. Cluster 3 consists of assays conducted against liver and ookinete stages. Separate clustering of assays against asexual and gametocyte stage at different compound concentrations again suggests general toxicity of assays conducted at higher compound concentrations. There are two possibilities why liver and ookinete stages cluster together. These two stages may be physiologically more similar to each other, or it may reflect the fact that these assays were conducted against P. berghei, compared to other assays that were conducted against P. falciparum.
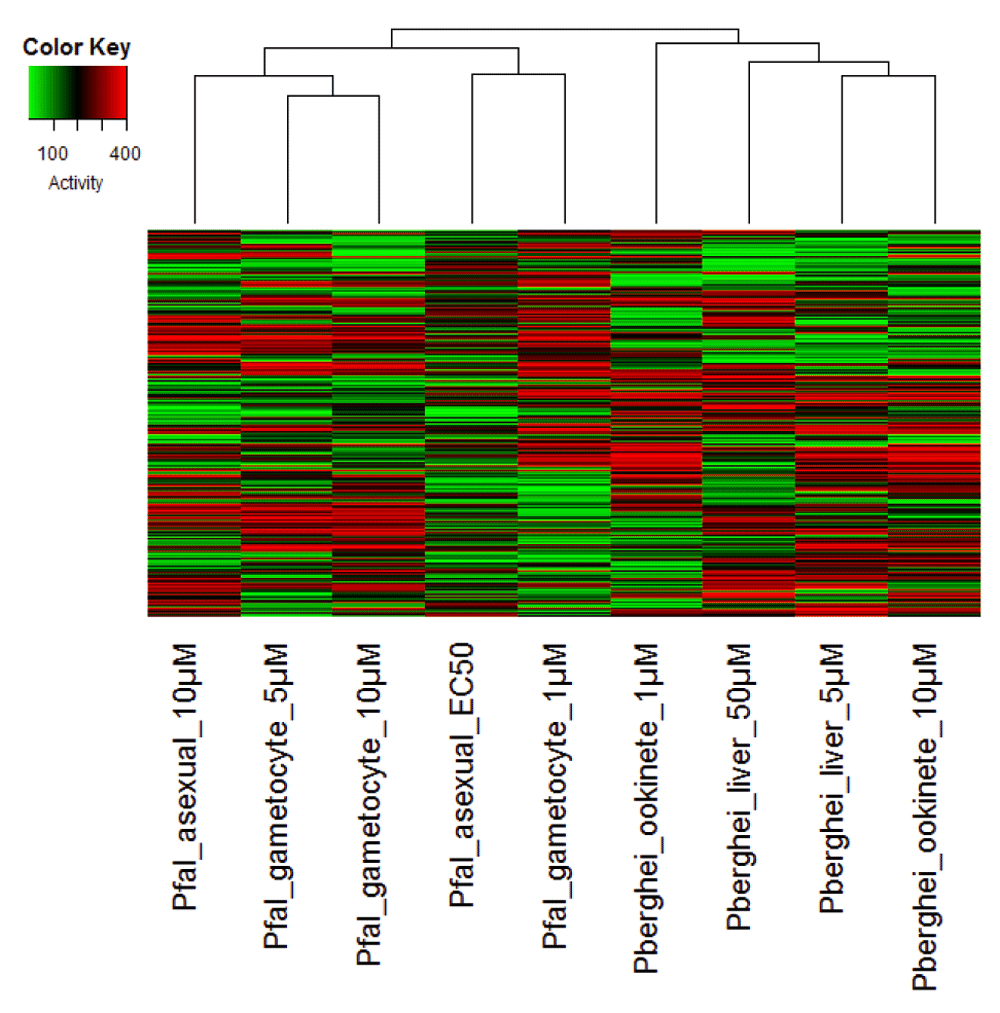
The three major clusters are evident that correspond to activity against P. falciparum at high concentrations (Cluster 1, leftmost), activity against P. falciparum at low concentrations (Cluster 2, middle), and possibly activity against P. berghei (Cluster 3, rightmost). Rank correlation values were used to create the distance matrix for the clustering. The color key shows the inhibitory activity of the compounds with a higher number representing a higher activity.
Given the possible confounding roles of compound concentration and Plasmodium species used in the screening, the prioritization of compounds that have pan-stage activity, but low host cytotoxicity, becomes difficult. We thus tested whether the Principal-Component Analyses (PCA) may be utilized to differentiate Plasmodium-specific activity from general toxicity. PCA analyses of the data from different Plasmodium stages and human cells lead to the identification of principal-components, which showed different properties with respect to general and specific activity. PC1 showed high correlation with assays conducted at higher compound concentrations and against a variety of cell types, including human cells (Figure 3), suggesting that PC1 is related to general toxicity. PC3, on the other hand, showed higher correlations with assays conducted at low compound concentrations, but negative or lower correlation with assays conducted at high compound concentrations in Plasmodium, against different pathogens and human cells (Figure 3), suggesting that PC3 is related to specific activity against Plasmodium across different stages. PC2 showed high positive correlation with the liver and ookinete stage assays, but showed negative correlations with asexual and gametocyte stage assays, suggesting that this component reflects activity against these two stages or against P. berghei in which these assays were performed.
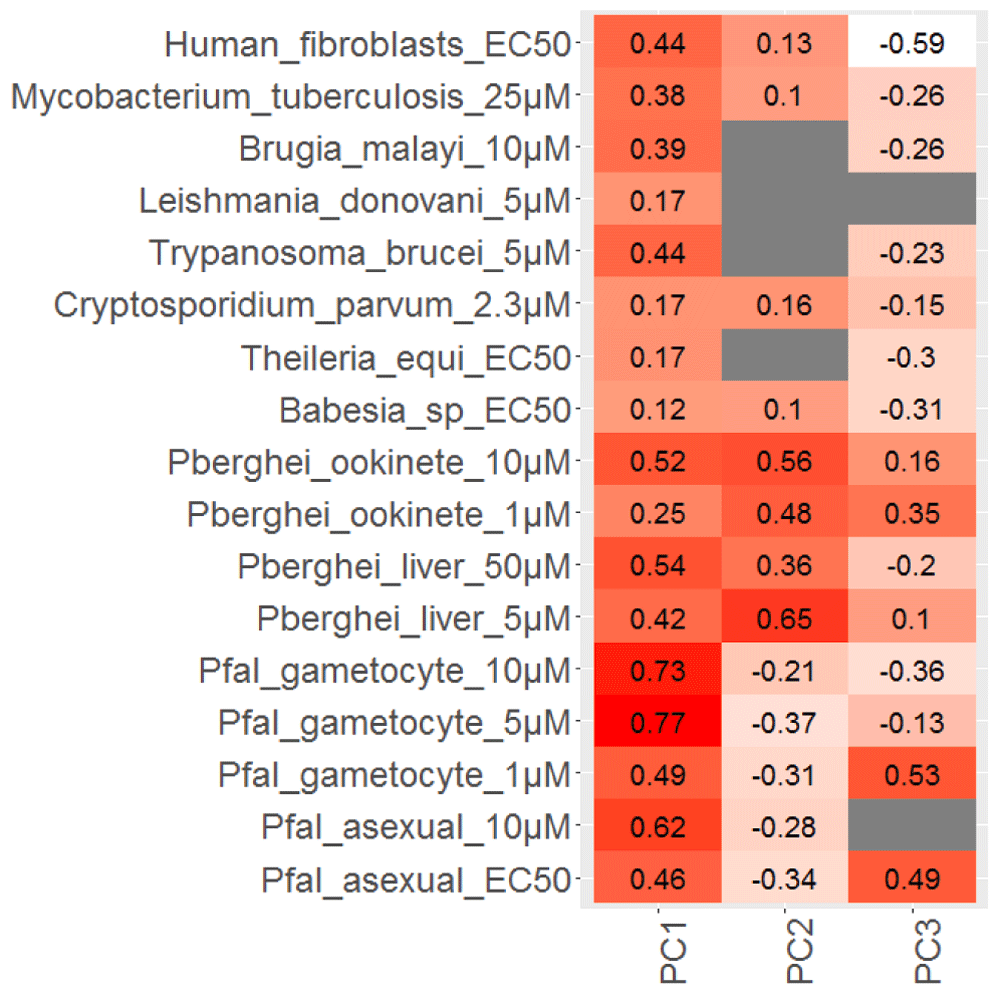
The three principal components PC1, PC2 and PC3 explained 30%, 16% and 13% of the variation in the data, respectively. PC1 showed positive correlation across different assays, suggesting that it reflects general toxicity. PC3 showed higher positive correlation only with assays conducted at lower compound concentration, but lower or negative correlation with assays conducted at higher compound concentration, other parasites and human cells, suggesting that it reflects pan-stage specific activity against Plasmodium.
In vitro resistance evolution has recently been attempted against 30 Malaria Box compounds with three independent lines for each compound11. We next tested whether the Plasmodium-specific activity or general toxicity estimated from the principal components might predict in vitro resistance evolution. Compounds for which resistance could not be developed showed significantly higher PC1 values (Figure 4A). These compounds also showed higher human toxicity (Figure 4B) and enrichment of probe-like compounds, which have chemical properties associated with higher non-specific activity9 (Figure 4C). These results suggest that general toxicity of compounds may lead to lower success in in vitro resistance evolution. On the other hand, high PC3 values were associated with higher likelihood of in vitro resistance generation (Wilcox test p = 0.02, not shown).
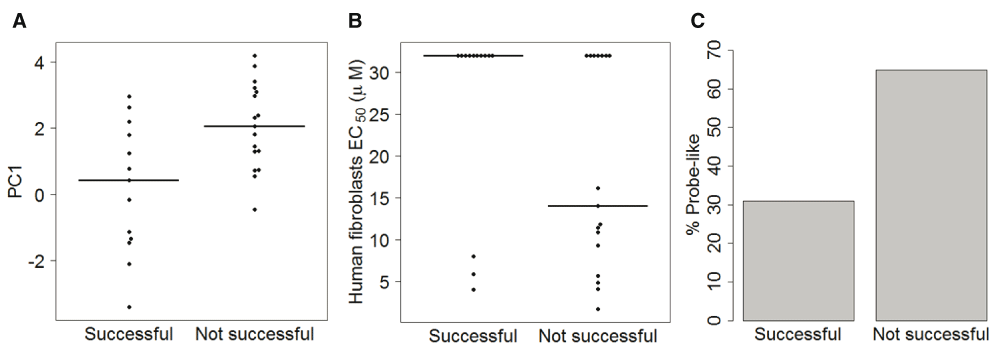
The in vitro resistance evolution was attempted for 30 Malaria Box compounds and was successful for 13 compounds11. Compounds for which resistance evolution was not successful showed (A) higher PC1 values (Wilcox p 0.004), (B) lower EC50 against human fibroblasts cells (Wilcox p 0.130), and (C) a higher proportion of probe-like compounds, as classified by Medicines for Malaria Venture9 (Fisher p 0.100).
In general, our results suggest that compounds that show high PC3 values should be prioritized for further development, including target identification by in vitro resistance evolution. Table 1 lists the top 20 Malaria Box compounds with the highest PC3 values. These compounds show high activity against multiple stages at a low concentration, but low activity against human cells. In total, 11 of these compounds show favourable oral bioavailability values. Some of these are active against other pathogens (Table 1). The values of three principal components for all Malaria Box compounds are available in Dataset 114.
These compounds show high activity against multiple stages at a low concentration, but low activity against human cells. The mouse oral bioavailability was obtained by measuring the plasma concentration of the compounds with a single high oral dose (140 μM/kg)10. Compounds with favourable plasma concentration (plasma Cmax > 1μg/ml) are indicated. Activity of compounds against other parasites is also indicated. The oral bioavailability data and compound activity data against other parasites was obtained from Van Voorhis et al.10. The PC3 values for all Malaria Box compounds are available in Dataset 114.
The wide availability of Malaria Box has catalysed a number of studies on these compounds10. Prioritization of compounds based on a large number of variables is not straightforward. Here, we analysed this data and found that a single variable (PC3) can capture most of the desirable compound properties: activity against multiple Plasmodium stages and low host cytotoxicity, thus greatly simplifying the task of compound prioritization. Our analyses suggest that screening at high compound concentrations can lead to general toxicity and thus should be avoided. Thus the idea that hits identified from multiple assays should be more confident10 needs to be considered carefully when hits are identified from high concentration assays. The consensus approach might lead to the selection of compounds with general toxicity.
We found significant correlation between activity against the asexual stage and the gametocyte stage of P. falciparum (Spearman rank correlation 0.43), which suggests that it might be easier to find compounds that have activity against both these stages, even though the two stages have different growth properties. The correlations between asexual stage with the liver and ookinete stages were low (Figure 1 and Figure 2). This could reflect different physiological states of liver and ookinete stages from asexual and gametocyte stages, but it might also reflect that liver and ookinete stage assays were performed in P. berghei, rather than P. falciparum. Thus, the development of higher throughput liver and ookinete stage assays in P. falciparum could be valuable. It is important to note that the correlation values that we report should be considered an underestimate, as inhibition values for assays against the same stage show large variability, e.g. median rank correlation among nine EC50 values against asexual stage of P. falciparum was 0.51. The possible reasons for this variability have previously been discussed10.
The difficulty in the evolution of in vitro resistance is considered a very desirable property of a compound12 given that a number of anti-malarial drugs are becoming less effective because of resistance generation13. Our observations suggest caution in interpreting the results of in vitro resistance evolution experiments. The failure to obtain resistance in vitro could be because of general toxicity of the compound on the erythrocyte hosts. Thus we suggest that the host toxicity of compounds should be thoroughly evaluated before conducting the labour-intensive in vitro resistance evolution experiments.
While we have prioritized compounds according to their pan-stage activity and low human toxicity, we would like to stress that compounds that show activity across pathogens and human cells may also be potential leads, if their toxicity could be managed. One possibility to reduce the toxicity of a compound is to identify its target in the parasite and its human ortholog, and utilize the three-dimensional structures of the compound with the target to modify the compound to increase selectivity. However, target identification of these compounds might be more difficult using in vitro resistance development.
This publication uses data on Malaria Box compounds, as reported and compiled by Van Voorhis et al.10 (DOI: 10.1371/journal.ppat.1005763.s002). The data on the in vitro resistance evolution is reported by Corey et al.11.
Dataset 1: PC1, PC2 and PC3 values for 400 Malaria Box compounds, DOI: 10.5256/f1000research.10121.d14257014
G.P.S. conceived and designed the study, performed the research and wrote the manuscript.
The work is supported by an Early Career Fellowship to G.P.S. by the Wellcome Trust/DBT India Alliance (IA/E/15/1/502297).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Suggestions and criticism on the manuscript from Dr. Amit Sharma and Ms. Preeti Goel from the author’s lab are gratefully acknowledged.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: Reviewer is one of the co-authors of Van Voorhis et al., 2016. That paper contains most of the biological data used in current analysis. However, reviewer does not feel this to have an influence in the output of my review.
Competing Interests: The paper uses the data from van Voorhis et al., 2016 as the basis of its data set. I am an author on that paper, and also on the original concept of the malaria box. I have never heard of, nor met the author however, and do not feel that this influences me in any significant way.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 18 Nov 16 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)