Keywords
diclofenac suppository, pain control, flexicystoscopy, office urology
diclofenac suppository, pain control, flexicystoscopy, office urology
The earliest reported use of flexible endoscope for examination of bladder neck was by Tsuchida and Sugawara1. It is now one of the most commonly performed diagnostic as well as therapeutic urologic interventions2. Pain associated with cystoscopy varies from patient to patient and there is continuous effort using various methods to reduce pain during and after the procedure to improve patient compliance for flexible cystoscopy. The majority of patients require local anesthesia or lubricant solution only but some patients may require intravenous sedation3 or inhalation analgesia (nitrous oxide)4. Factors contributing to severity of pain include: lubrication, use of topical anesthesia and duration of cystoscopy5–7 but the available evidence for best practice in terms of treatment is continuously evolving8. The important issues regarding the correct use of intra-urethral gels are, for the most part, left to individual preference9. Effect of different intra-urethral gels, their dosage, temperature and time of instillation on pain perception has been evaluated in literature. In a randomized control trail, 2% lidocaine gel in two different doses (10 and 20 ml) and plain lubricating gels were found to be equally effective for pain control during flexible cystoscopy (p=0.406)10. Pain perception with use of lidocaine versus plain lubricating gel is less as reported in a meta-analysis by Aaronson et al.11 while another meta-analysis by Patel et al. has reported no statistical difference among the two gels for pain control12. In a study by Komiya et al., oral zaltoprofen has been used as pre-emptive analgesia for rigid cystoscopy and it has been proved to provide better pain control than 2% lidocaine gel alone (11.35 versus 13.69 with a difference of pain score -2.8, p-value 0.0087)13. Intra-rectal diclofenac suppository administration used by Irer et al. has a proven role to reduce pain and improve patients’ tolerance of trans rectal ultrasound-guided prostate biopsy14.
Diclofenac is an anti-inflammatory drug with local and systemic effects; the local effects include reducing the impact of pain mediators. The diclofenac suppository in comparison to the oral has a rapid onset and a slower rate of absorption. The maximal plasma level Is reached within 2 hours, and is maintained for up to 12 hours and that forms the basis of using suppository rather than oral NSAID in our study15. In the current study we have attempted to assess the use of diclofenac suppository as a pre-emptive analgesia during flexible ureteroscopy.
The Ethical Review Committee of the Aga Khan University and the Clinical Trial Unit approved the study protocol. The study was registered at www.clinicaltrials.gov (ClinicalTrials.gov identifier: NCT01812928). This trial was conducted at the surgical day care unit from February 2013 to July 2013.
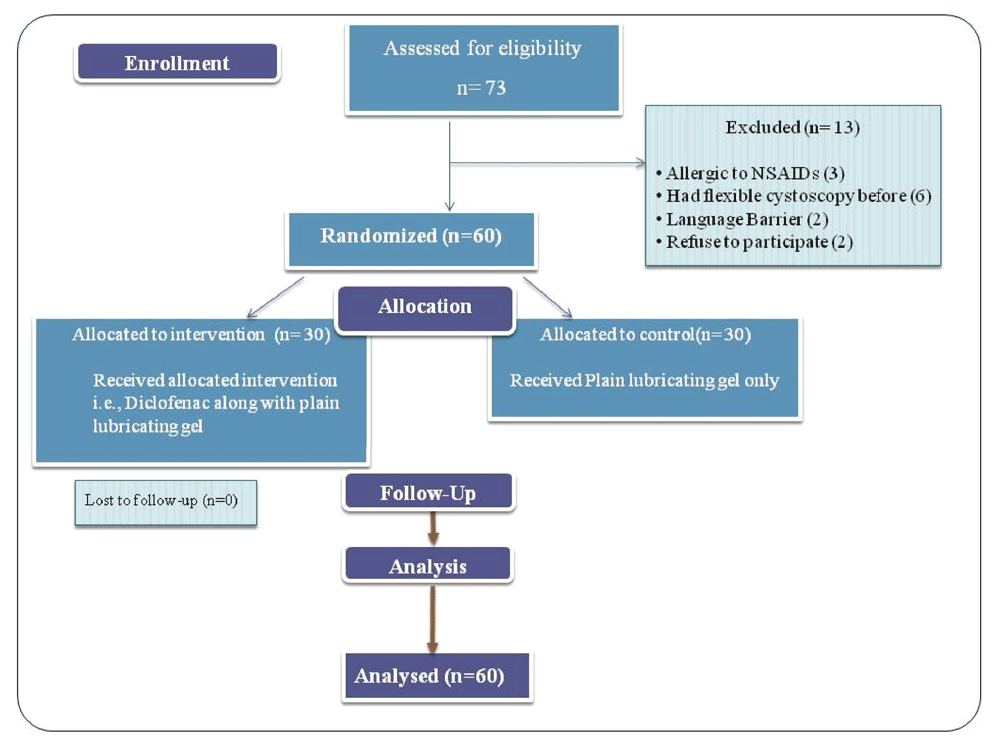
Details of recruitment and flow of study has been demonstrated as CONSORT flow diagram. (Figure 1). The principal investigator of this study obtained the written consent from all the qualified patients before randomization. All male patients of 18 years of age and older with indication for flexible cystoscopy, were assessed for recruitment in the trial. We included all adult males who attended for evaluation of hematuria or lower urinary tract symptoms and those for removal of double J ureteral stent. All patients undergoing the procedure had a urinalysis and culture to exclude UTI. Patients were requested to empty the bladder immediately prior to the procedure or within 30 minutes. Prior to the procedure, patients were explained the visual analog scale (VAS; score zero means no pain and 10 means worst pain). Eligible patients were randomized by a computer-generated list and sealed envelopes. Patients were randomized into either Group A (those patients who received diclofenac suppository prior to procedure) or Group B (those patients who did not receive diclofenac suppository prior to procedure) using a web-based random number generator (RANDOM.ORG, Dublin, Ireland; https://www.random.org). Diclofenac suppository (100 mg) was administered rectally 1 hour prior to the procedure in the pre-operative area. Both groups received 10 ml of plain lubricating gel immediately before the procedure for the purpose of lubrication.
The procedure was performed at the surgical day care unit in supine position by a consultant urologist or senior urology resident (residency year 5 and 6) that was blinded to the randomization group. A second resident immediately following the procedure, collected data (pain score) in the operating room. The VAS consists of a straight line with the endpoints defining extreme limits such as ‘no pain at all’ and ‘pain as bad as it could be’16. The investigator was blinded to the group (independent assessor). Operative time was recorded from the operating room time log. Pre- and post-procedure pulse rate and blood pressure were recorded for all participants.
Data was analyzed using SPSS™ version 17.0. Results were described in terms of mean and standard deviation for age, duration of procedure and pain score while frequency and percentage were mentioned for categorical variables. The student t-test (independent samples, one-tailed) was used to determine statistical significance of VAS for pain between group A and B. Confounder and effect modifiers i.e. age, level of the person performing procedure, indication for procedure and duration of procedure were analyzed using linear regression analysis. p-value of <0.05 was considered as statistically significant.
Seventy-three patients were evaluated for inclusion in the study. A total of sixty patients were recruited in the trial and analyzed. The mean age was 46.75 ± 16.12 years (IQR: 18–80). The most common indication for flexible cystoscopy was removal of double J Stent (n= 38, 63.3%), others were for evaluation of hematuria (16, 26.7%) and lower urinary tract symptoms (6, 10%). Year 5 and 6 urology residents performed the majority of the procedures (n= 56). Mean duration of the procedure was 5.52 ± 2.13 minutes (IQR: 2–10 minutes). On the 11 point VAS the mean pain score was 3.63 with a standard deviation of 1.46 for the entire group (IQR: 0 – 7). The highest pain score was of 7 on VAS reported by only one patient from group B.
The mean age of the patients in groups A and group B were 48.53 ± 17.81 years and 44.97 ± 14.31 years respectively and there was no statistically significant difference (p= 0.53). The pre-procedure pulse and systolic blood pressures were comparable in both groups. Mean duration of procedure in group A was 5.76 ± 2.25 minutes and in group B was 5.28 ± 2.00 minutes. This difference in duration was not statistically significant (p=0.82). Indications for the procedure and level of operating surgeon were also comparable between the groups.
Mean pain score in group A was 3.16 ± 1.53 and in group B was 4.10 ± 1.24. This difference in the mean pain score was found to be statistically significant (p= 0.012). None of our patients required additional analgesia in either group. The difference in post-procedure pulse rate was found to be statistically significant (p=0.01) between groups however no statistically significant difference (p=0.15) was observed in systolic blood pressure between two groups (Table 1).
Linear regression analysis was performed. None of the confounding factors (including age, indication for procedure, level of operating surgeon and duration of procedure) was found to have significant impact on the outcome parameter (r2 = 0.026, standard error of estimate= 1.479; Table 2).
Model summary (a)
| Model | R | R square | Adjusted R square | Std error of the estimate |
|---|---|---|---|---|
| 1 | 0.163* | 0.026 | -0.026 | 1.47961 |
Duration of procedure (b)
We examined the effect of pre-emptive analgesia on pain perception during flexible cystoscopy and found out that diclofenac suppository significantly reduces pain when administered as pre-emptive analgesia before flexible cystoscopy.
Randomized studies by Patel et al.12 regarding use of lidocaine versus plain gel, which included 817 patients, showed that intra urethral lidocaine gel had no statistical effect on pain on a 100-point VAS scale (95% CI, -9.6 to 0.385). This meta-analysis challenged the commonly held belief among clinicians that intra urethral lidocaine gel is more efficacious than plain gel for decreasing pain during flexible cystoscopy12. In contrast to the findings of Patel et al.12, Cornel et al. observed slightly less pain (statistically non significant) in the test group and pain perception was the same between patients with past experience of cystoscopy and initial cystoscopy17. To avoid this bias, we kept very strict inclusion criteria and excluded all the patients with previous experience of flexible cystoscopy.
The present study has demonstrated significant reduction in pain perception during flexible cystoscopy in male patients with use of diclofenac suppository as pre-emptive analgesia. Sample size was calculated a priori to detect the effect, according to Lwanga et al.18 We followed stringent criteria for enrollment of patients in this trial to eliminate confounding factors for pain. Computer generated sequences were used for randomization in order to give equal chance of being selected in either group to all recruited patients.
Flexible cystoscopy is often performed repeatedly in particular during the follow up of urothelial cancer. As repeated cystoscopy did not increase the patient's tolerability to pain associated with cystoscopy, Muezzinoglu noted the need for more effective anesthesia to improve tolerability during the procedure and maintain quality of life of the patients under long-term follow-up with repeated cystoscopies19. Till date various techniques have been used to ameliorate the perception of pain during flexible cystoscopy. Use of NSAID as pre-emptive analgesia has been tested for various surgical procedures20,21. Komiya and co-workers examined the effect of anti-inflammatory drug (NSAID) zaltoprofen that inhibits the generation of prostaglandins as well as the pain induced by bradykinin during rigid cystoscopy13. The mean age of the patients in their study was 69.3+/- 8.2 (range: 41–83) while in our study we had relatively younger study subjects (mean age+/- SD, range: 46.75+/-16.1 years, 18–80 years) who are presumably more anxious with lower pain threshold. Despite this fact, diclofenac suppository significantly improved the pain perception and proved to be effective regardless of age on regression analysis. Another matter of debate is the statistical method used in the study by Komiya et al.13 where they used a “one sample Wilcoxon test” for comparing the two groups which is rather an inappropriate test to demonstrate the effect. The one-Sample Wilcoxon signed-rank test is a non-parametric alternative to a one-sample t-test. The test determines whether the median of the sample is equal to some specified value. Data should be distributed symmetrically about the median. In the present study we have used regression analysis, which is a more stringent method to demonstrate the effect.
In our study, we used diclofenac suppository as pre-emptive analgesia. The pharmacokinetics of the suppository form is quite different from the orally administered agent. It acts as an anti-inflammatory drug both locally and systemically, by minimizing the effects of local mediators involved in the pain response. Diclofenac has been marketed internationally since 1973 and is currently available in oral, rectal, parenteral and topical preparations15. The efficacy of the diclofenac suppository is due to more rapid onset of effect, and a slower rate of absorption (it takes approximately 4.5 hours for complete absorption) than oral enteric-coated tablets. The maximal plasma level is attained within 2 hours, and it is maintained for up to 12 hours15. The terminal half-life of diclofenac in plasma is 1 to 2 hours. The major route of excretion is the urine (~60%) and a small percentage through bile in the feces22. Its role has proven to be effective for pain control during trans rectal ultra sound guided prostate biopsy in study by Haq et al.23. In a case control the investigators noted that it is a simple and safe method. While Irer et al.14 showed additional benefit of using lidocaine gel for pain control during the same procedure but statistical significance of this study is in question due to its smaller sample size.
In the present study, appropriate sample size, stringent criteria for recruitment, computer generated randomization, proper statistical methods and analysis has increased the scientific rigor. This was not a placebo controlled as various per rectally medications or “dummy drugs” may have some local inflammatory effect.
Intra rectal diclofenac suppository is a simple and effective method to reduce pain during flexible cystoscopy regardless of age. We recommend its routine use for better tolerability of pain and to increase patient’s compliance.
F1000Research: Dataset 1. Raw data for ‘Effect of diclofenac suppository on pain control during flexible cystoscopy-A randomized controlled trial’, 2016, 10.5256/f1000research.9519.d14526824
M Nadeem: conception, study conduct, data analysis, writing manuscript.
MH Ather: conception and study design, writing manuscript.
This work was funded by a University of Research Council Grant (70823) to HA.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 08 Dec 16 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)