Keywords
Arsenic, Benzo-α-pyrene, Biocides, Bisphenol A, Cadmium, Cigarette smoke, Combustion Pollutants, Diethylstilbestrol, Egg Laying, Egg Hatching, Egg Viability, Endocrine Disruptors, Fenthion, Nicotine, Tributyltin, Triclosan
Arsenic, Benzo-α-pyrene, Biocides, Bisphenol A, Cadmium, Cigarette smoke, Combustion Pollutants, Diethylstilbestrol, Egg Laying, Egg Hatching, Egg Viability, Endocrine Disruptors, Fenthion, Nicotine, Tributyltin, Triclosan
Teratogens are agents that negatively impact reproduction and embryonic development and include radiation, maternal infections, pharmaceuticals, and chemicals (Wilson, 1973). Numerous chemicals act as teratogens that adversely affect human health, with the time period of exposure as a critical factor determining teratogen susceptibility. For instance, maternal and prenatal teratogen exposure is associated with birth defects, spontaneous abortion, and stillbirth, and sometimes cancer in the reproductive tract of progeny (Reed et al., 2013; Sanders et al., 2014; Wigle et al., 2008). Previous studies demonstrated that acute, high-dose teratogen exposure causes reproductive decline, but the long-term ramifications of low dose teratogen exposure during early development later in life remain unknown (Allard & Colaiácovo, 2010; Parodi et al., 2015). In this study, we used egg-laying, hatching, and offspring viability assays phenotyping screen throughout early development after exposing Caenorhabditis elegans to sub-lethal doses from in three classes of teratogens, including biocides, endocrine disruptors, and combustion pollutants, to detect impacts on reproductive phenotype in adults.
In addition, identification of the time frame, yielding the maximal egg-laying, after teratogen exposure, is critical for behavioral and developmental experiments requiring an aged-matched offspring population. The results of our study guide experimental procedures to obtain the requisite population size for subsequent experiments employing 3 hours egg-laying time window to achieve developmentally synchronous, aged-matched offspring population for subsequent experiments assessing long-term effects.
N2 ancestral Bristol strain of C. elegans (CGC, Minneapolis, MN, USA) were maintained on NGM lite plates (N1005, US Biologicals, Salem, MA, USA) with bacterial lawns at 25±0.5°C for all experiments. Bacterial lawns were grown on Petri dishes (10, 60, 100 mm Corning, USA) overnight using 10, 20 or 60 µl of 1X E. coli OP50 (CGC, Minneapolis, MN, USA; 1X=OD600=8.0×108 cells/mL).
Teratogens, except for cigarette smoke extract, were purchased from Sigma Aldrich (St. Louis, MO, USA): tributyltin-chloride (0.1 µM, T50202), cadmium-chloride (0.5 µM, C2554), benzo-α-pyrene (0.5 µM, B1760), nicotine (0.5 µM, N-3876), bisphenol-A (10 µM, 239658), diethylstilbestrol (10 µM, D4628), arsenic(III) oxide (0.5 µM, A1010), triclosan (0.1 µM PHR1338), fenthion (0.1, 1 µM, 36552). Cigarette smoke extract (0.1 µM) was purchased from Murty Pharmaceuticals (0.1 µM, Lexington, KY, USA). Stock solutions of teratogens were made in DMSO or water, and final concentrations were adjusted to contain 0.01% DMSO (ACS grade, Fisher Scientific, USA). Sub-lethal doses were chosen based on preliminary studies (unpublished McIntyre, Killeen & Marín de Evsikova).
The experimental design for all studies is depicted in Figure 1. A mixed population of worms was used to prepare a developmentally synchronized population at L1 larval stage (Stiernagle, 2006). All plates were coded to prevent experimenter bias. Larvae were transferred onto vehicle or teratogen exposure plates (with bacterial lawns) and cultivated for 48 hours at 25°C (Figure 1). Adult worms from each group were washed 3 times in M9 buffer and transferred into a well in the first row of a 24-well NGM plate. These worms were moved to an adjacent well every three hours for 12 hours. Laid eggs, and the following day, hatched embryos were counted. Embryo viability was determined as the ratio of hatched larvae to number of eggs. Experiments were repeated six times (n=60 worms/group, yielding N=660 worms), albeit fenthion, which was repeated twice at 1 µM (n=20), and after unexpectedly curtailed egg-laying, the dose was decreased to 0.1 µM (n=40). Statistically significant differences were determined after outlier analysis using SPSS v. 23 software (IBM, USA) by ANOVA followed by post hoc or nonparametric (Χ2 or Fisher’s Exact Tests) for fecundity, fertility, eggs laid, hatched larvae, and embryo viability among all groups with significance set at p<0.05.
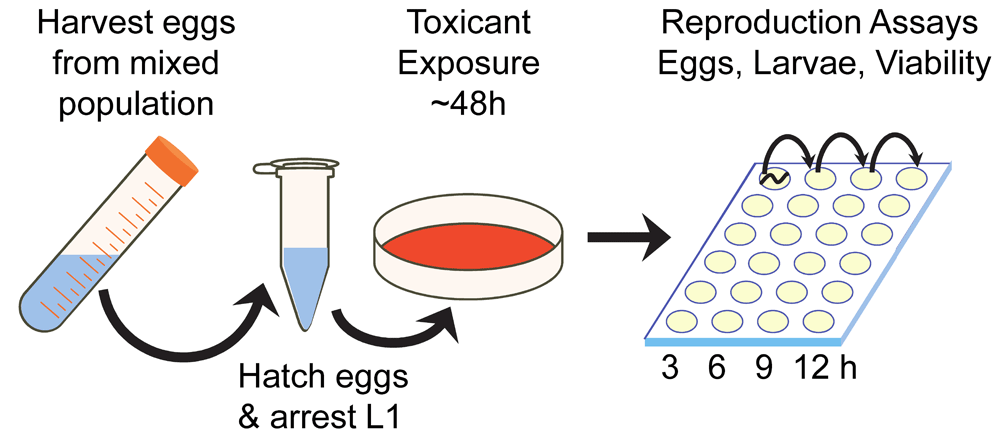
An age-synchronized population of C. elegans L1 larvae was exposed to 10 known teratogens at sub-lethal, micromolar concentrations for 48 hours. Gravid adult worms were transferred to pure NGM with bacterial lawns and the number of eggs laid was recorded every 3 hours up to 12 hours. Hatched larvae counted the following day.
Fecundity and fertility, measures of reproductive fitness of the organism, were decreased after worms were exposed to the biocides, triclosan and fenthion, and combustion pollutant, benzo-α-pyrene (BAP; Χ2= 11.27, 5.21, and 83.1, p<0.05, respectively, Figure 2A & B). Despite the overall detrimental effect on fecundity and fertility, the initial temporal pattern of egg laying was increased by some teratogens. The cumulative amount of eggs laid, irrespective of teratogen or vehicle, increased over time except for 1 µM dose of fenthion (Figure 3A, F(3,162)=119, p<0.05). Furthermore, chronic larval exposure to some low dose teratogens, such as nicotine, cadmium, and tributyltin, increased egg laying and cumulatively produced more eggs than vehicle control (Figure 3A). Unexpectedly, many teratogens, except for arsenic and benzo-α-pyrene, produced more eggs during the 0–3 hours interval compared to vehicle (Figure 3B), although this interval had the overall lowest yield of eggs. While the greatest amount of egg-laying occurred during the 9–12 hours compared to the 0–3 hours interval, no differences in the amount of eggs laid were detected among the intervals at 3–6 hours, 6–9 hours, and 9–12 hours (p>0.05). Hatched larvae, unlike eggs, did not increase at any interval after teratogen exposure (Figure 4B, F(33,162)=1.051) but total hatched larvae increased cumulatively by 12 hours (F(3,162)=24.8, p<0.05). Despite these increases, egg quality did not improve after teratogen exposure as overall embryo viability was similar across groups and time points (Figure 5A F(33,162) = 1.015, P>.4) with lowest viability at the first interval (Figure 5B) albeit not significant.

Effects of teratogens on fecundity (A) and fertility (B). The overall percentage of worms that laid eggs shown as fecundity and the overall percentage of viable and dead eggs as a measure of fertility. Asterisks indicate P<0.05.
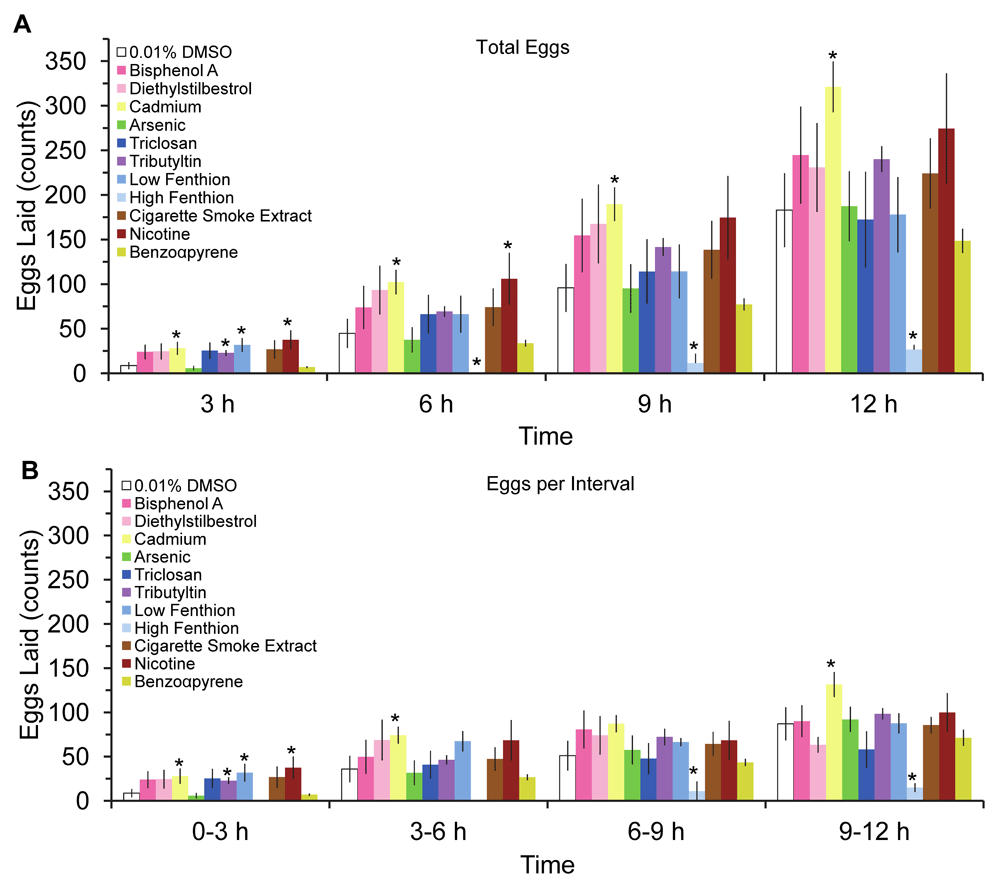
Temporal pattern of (A) cumulative eggs laid at 3, 6, 9 and 12 hours post-exposure, and (B) rate of eggs laying in 3 hours intervals (mean ± sem). Asterisks indicate P<0.05.
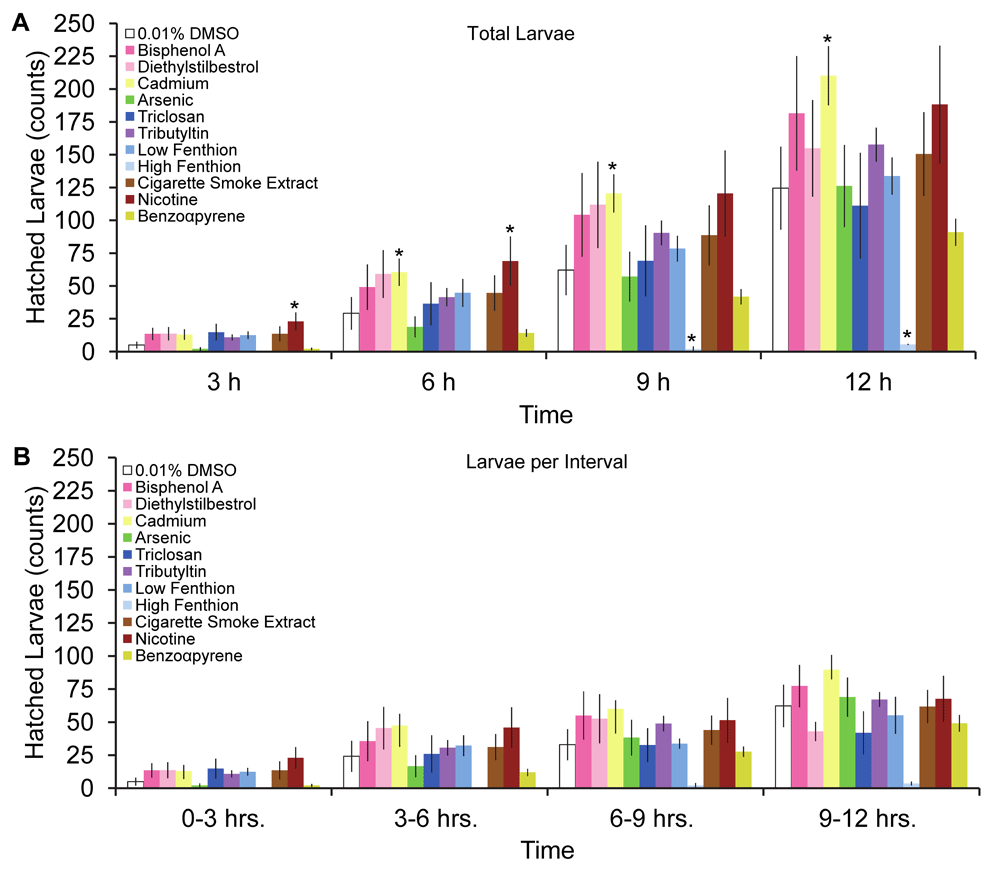
Temporal pattern of (A) cumulative hatched larvae from eggs laid at 3, 6, 9 and 12 hours post-exposure to teratogens and (B) number of hatched larvae per teratogen group or control in 3 hours intervals (mean ± sem). Asterisks indicate P<0.05.
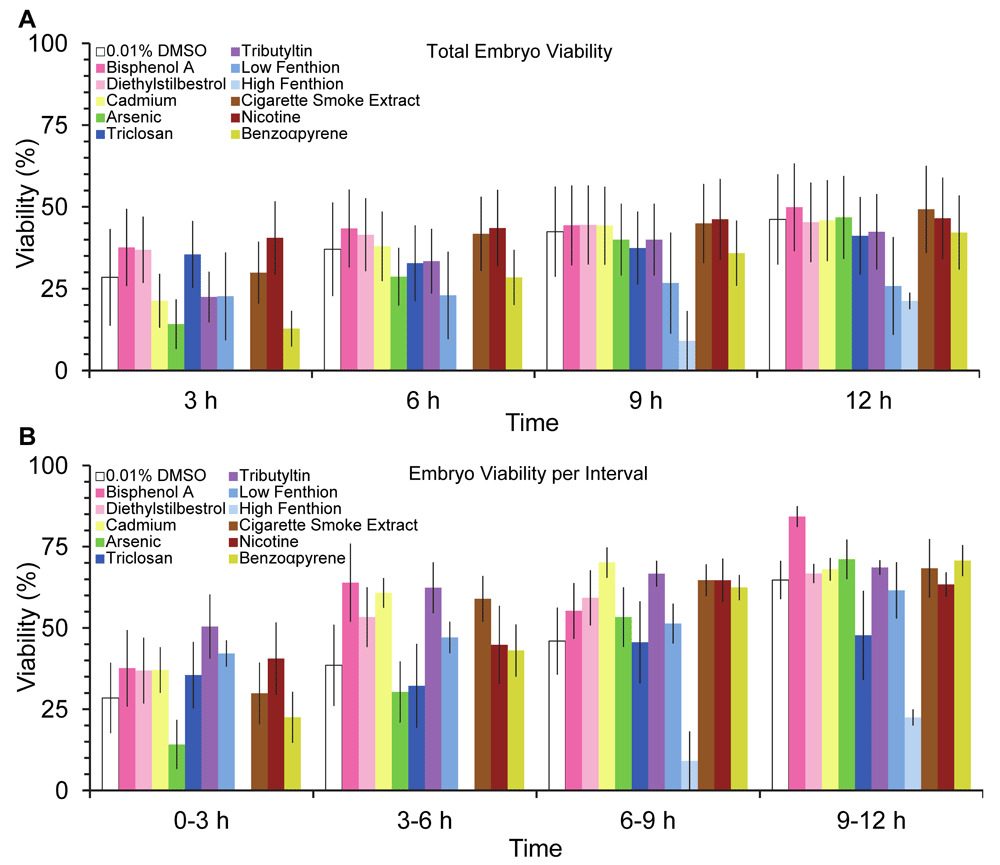
Temporal pattern of (A) overall embryo viability at 3, 6, 9 and 12 hours post-exposure to teratogens and (B) embryo viability at 3 hours intervals. Embryo viability calculated as the ratio of hatched larvae/eggs laid X 100% (mean ± sem). Asterisks indicate P< 0.05.
Early larval exposure to low dose teratogens alters egg-laying without improving embryo viability at later time points, which result in some overall detrimental impacts on fecundity and fertility. An improvement in embryonic viability at the latter time intervals was expected because adult hermaphrodites replenish their entire gonad every 6.5 hours (Hirsh et al., 1976), which implies that eggs laid during or after the 6–9 hour interval hours would have not been exposed to teratogens and egg-laying and egg quality should have increased at the latter two intervals compared to earlier intervals of 0–3 hours and 3–6 hours. While egg-laying improved during at the 9–12 hours interval, egg quality did not improve compared to either 0–3 or 3–6 hours intervals, as measured by embryo viability. Due to specifics of gametogenesis in C. elegans, it is possible that teratogens may affect egg quality by altering sperm cell quality (Hirsh et al., 1976). It is also possible that chronic exposure to teratogens may be exacerbated by their extended bioactivity, or in some cases, through actions of their metabolites. These results indicate greater egg harvests are necessary to obtain an offspring population to further assess long-term teratogen effects on offspring.
This procedure using C. elegans not only identifies sub-lethal teratogen concentrations with potential long-term effects upon egg laying and quality, it provides the experimental platform to expand knowledge to prevent birth defects by developing interventions to ameliorate chronic subtoxic, teratogenic exposures upon the embryo, and is an initial step towards designing and testing safe therapeutics to be used before and during gestation. Developing phenotyping screens with simple organisms, such as C. elegans, is an efficient way to identify putative teratogens with potential long-term effects to further knowledge on the underlying developmental processes susceptible to environmental insults, and reveals the basis of how environment affects and shapes development.
F1000Research: Dataset1. Dataset of screening sub-lethal teratogens on egg laying, hatching and viability in adult C. elegans during larval development, 10.5256/f1000research.8934.d124253 (Killeen & Marín de Evsikova, 2016).
AK and CM conceived the study and designed the experiments. AK executed and analyzed the experiments. AK and CM drafted the manuscript. Both authors were involved in the revision of the draft and agreed to the final manuscript.
C. elegans N2 ancestral strain and E. coli OP50 strain was provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
The authors thank Shpetim Karandrea for coding the groups to collect the data blind with respect to teratogen or control exposure.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 28 Dec 16 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)