Keywords
Drosophila midgut, Wingless, Intestinal stem cells, Homeostasis
Drosophila midgut, Wingless, Intestinal stem cells, Homeostasis
Apc Adenomatous polyposis coli
Arm Armadillo
BBS Borate buffered solution
EB Enteroblast
EC Enterocyte
EE Enteroendocrine
Esg Escargot
FLP Flippase
FRT Flippase recognition target
Fz Frizzled
GFP Green fluorescent protein
HRP Horseradish peroxidase
ISC Intestinal stem cell
MARCM Mosaic analysis with a repressible cell marker
Pros Prospero
RFP Red fluorescent protein
Su(H) Suppressor of hairless
TA Transit amplifying
TARGET Temporal and regional gene expression targeting
TS Temperature sensitive
Tub Tubulin
Wg Wingless
The discovery of intestinal stem cells (ISCs) in the adult Drosophila midgut established an attractive model for the study of tissue homeostasis (Micchelli & Perrimon, 2006; Ohlstein & Spradling, 2006). The Drosophila midgut displays similarities with the mammalian intestine in various aspects, such as cell composition and regulatory mechanisms (Micchelli & Perrimon, 2006; Ohlstein & Spradling, 2006). Like the mammalian intestine, the Drosophila adult midgut consists of a tubular, monolayered epithelium lining the length of the midgut, surrounded by the basement membrane and two layers of visceral muscles (Micchelli & Perrimon, 2006). The ISCs are dispersed among the differentiated cells throughout the enteric epithelium (Micchelli & Perrimon, 2006; Ohlstein & Spradling, 2006).
Two fully differentiated cells types populate the Drosophila midgut: large, polyploid enterocytes (ECs), the main absorptive cells in the epithelium, and small, diploid enteroendocrine (EEs) cells, the secretory cell type. ECs/EEs come from the differentiation of an intermediate cell type, called enteroblasts (EBs). EBs are conceptually similar to the Transit Amplifying (TA) cells in mammals, though unlike the TA cells, EBs do not divide before terminal differentiation. Lineage analysis has shown that ISCs undergo asymmetric divisions as well as symmetric self-renewal and symmetric differentiation (de Navascués et al., 2012; O'Brien et al., 2011), resulting in homeostasis by population asymmetry (de Navascués et al., 2012; Klein & Simons, 2011).
Wnt signalling plays an indispensable role in the regulation of mammalian ISCs. In the mammalian intestine, Wnt signalling is crucial in the maintenance of stem cell crypts (Barker et al., 2007; Barker et al., 2009; Van der Flier & Clevers, 2009; Van der Flier et al., 2007). Wingless (Wg), the Drosophila homologue of Wnt1, is expressed in the adult midgut, and Wg signalling has been shown to play a crucial role in tissue regeneration (Cordero et al., 2012). Studies have shown that stress-induced epithelial Wg production from EBs is essential for ISC proliferation during tissue renewal, but not required for midgut maintenance under homeostatic conditions (Cordero et al., 2012; Micchelli & Perrimon, 2006). However, other works reported that Wg signalling is required for ISC self-renewal: reduced proliferation and premature differentiation occur as a consequence of inhibiting downstream Wnt signalling (Lin et al., 2008). By contrast, a separate study proposed that the loss of Drosophila adenomatous polyposis coli, Apc, does not affect ISC self-renewal nor EB cell fate specification (de Navascués et al., 2012; Lee et al., 2009; Lin et al., 2008; O'Brien et al., 2011). Lee et al. (2009) showed that Apc is required for midgut homeostasis and regulates ISC proliferation, and its absence leads to midgut hyperplasia and multilayering. Moreover, a recent work indicated that Wg signalling in ECs act non-autonomously to prevent ISC proliferation (Tian et al., 2016). Thus the exact function of Wg signalling on ISC proliferation and EB differentiation remains controversial.
Reports have shown wg expression in the epithelium of the foregut-midgut and midgut-hindgut boundaries (pylorus) (Lee et al., 2009; Singh et al., 2011; Takashima et al., 2008; Tian et al., 2016), and also in the visceral muscle (Cordero et al., 2012; Lin et al., 2008; Tian et al., 2016). The expression of wg at the midgut boundaries may take part in regulating foregut and hindgut development (Lee et al., 2009; Singh et al., 2011; Takashima et al., 2008), though its function during adulthood is unclear. Moreover, the function of Wg emanating from the muscle has not been examined.
Using a Wg-responsive reporter transgene, frizzled3-RFP (fz3-RFP) (Olson et al., 2011), it was observed that fz3-RFP is expressed in gradients in the midgut epithelium, comprising both ISCs/ECs, and coinciding with regional boundaries (Buchon et al., 2013; Tian et al., 2016). At the pylorus, the gradient of fz3-RFP correlates with other gene expression gradients and the morphology of enterocytes, suggesting that Wg signalling activity affects gene expression and enterocyte architecture (Buchon et al., 2013; Lin et al., 2008; Micchelli & Perrimon, 2006; Ohlstein & Spradling, 2006).
Here, we investigated the expression and localisation of Wg, several components of the signalling pathway, and the signalling reporter, fz3-RFP. Our results showed a Wg concentration gradient at the adult Drosophila posterior midgut, in agreement with previous reports (Tian et al., 2016). The Wg protein is produced from two sources, one at the pylorus, and the other from the epithelial cells themselves. We show that in unchallenged conditions wg is sporadically expressed within the midgut epithelium, without any apparent spatial or temporal pattern. Using the stability of Arm as an instantaneous readout of Wg signalling, we further showed that the epithelial cells respond to Wg signalling in a similarly stochastic manner. In this study, we describe in detail the expression and activity patterns of Wg signalling in the Drosophila midgut, as well as depicting the response of the midgut tissue to Wg signalling.
To observe the pattern of wg expression in the adult Drosophila posterior midgut (regions R4 and R5, (Buchon et al., 2013)), we used the Wg antibody and several wg transcriptional reporters. An enhancer trap insertion in the wg locus (wg-Gal4 > UAS-GFP) (Pfeiffer et al., 2000) coincided with the anti-Wg antibody, and revealed that wg is highly expressed at the pylorus (Figure 1A–B), in agreement with previous reports (Singh et al., 2011; Takashima et al., 2008). We did not observe Wg expression in the midgut cells adjacent to the pylorus, as recently reported (Tian et al., 2016). Our observation was further confirmed with a wg-lacZ enhancer trap insertion (wg02657), which showed a strong pyloric expression (Figure S1). To evaluate whether this Wg protein could be emanating from the pylorus, or instead came from areas of wg expression in the midgut that were not recapitulated by the enhancer trap insertions, we used wg-Gal4 to drive the expression of a GFP-tagged Wg (wg-Gal4 > UAS-GFP:wg) (Packard et al., 2002). We observed GFP:Wg accumulation at the pylorus, and a decreasing gradient from the pylorus towards the anterior end of the midgut (Figure 1C–D and Figure S2). Notably, GFP:Wg diffused from the pylorus much further into the posterior midgut than into the hindgut epithelium. Interestingly, Wg travels in the midgut tissue to a distance longer than the width of the third larval instar imaginal wing primordium, where a Wg gradient is also established (Neumann & Cohen, 1997).
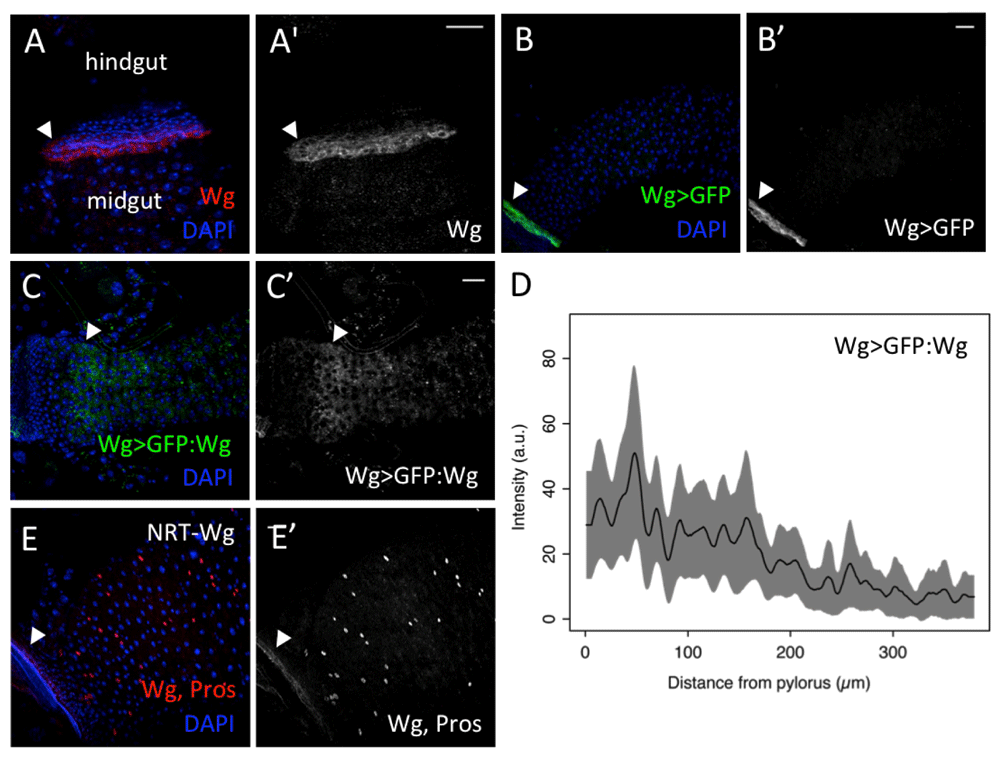
(A–A’) Anti-Wg (red, A; grey, A’) (B–B’) wg-Gal4, UAS-GFP (green, B; grey, B’) show high levels at the pylorus (arrowheads). (C–C’) wg-Gal4, UAS-GFP:wg (green, C; grey, C’) shows signalling gradient with high levels at the pylorus (arrowheads). (D) Intensity values of wg-Gal4, UAS-GFP:wg along thirteen parallel lines (as in the arrow in C’), averaged and smoothened with a Gaussian filter (5 µm wide) (black line). The corresponding raw data is represented in Figure S2. The limits of the grey area mark one standard deviation from the average value. (E–E’) Flies expressing only NRT-Wg showed high Wg signals (anti-Wg) (red, E; grey, E’) at the pylorus (arrowheads). EEs are marked by nuclear anti-Prospero staining (red, E; grey, E’). Scale bars: 25 μm.
We also looked at flies expressing a membrane-tethered form of Wg (NRT-Wg) from the endogenous wg locus (Alexandre et al., 2015). Anti-Wg again showed high Wg signals at the pylorus, with no obvious signalling gradient (Figure 1E,E'). These results suggest that the pylorus is the main source of Wg signal for the adult Drosophila posterior midgut, and that the Wg ligand can diffuse from this region, forming a gradient.
Our results so far indicate that most of the Wg protein in the posterior midgut comes from the pylorus. However, we still detected faint Wg antibody staining in midgut areas anterior to the lowest point of the Wg gradient, and we also observed low activity of wg transcriptional reporters (Figure 1). To ascertain whether there was wg expression in the homeostatic midgut epithelium, as reported during regeneration (Cordero et al., 2012), we made use of the wg{KO; Gal4} line. In this line, the wg locus was edited to express Gal4 instead of Wg (Alexandre et al., 2015), and therefore is expected to accurately reproduce the expression of wild-type wg.
To amplify the signal of expression, wg{KO; Gal4} was crossed to UAS-Flp, act<stop<lacZ; tub-Gal80ts (wg{KO}ts>Flp, act<<lacZ). After switching to the restrictive temperature, Gal4 activates and over time all Wg-producing cells and their offspring will be strongly labelled with LacZ, even at minimal levels of wg expression. When the wg{KO}ts>Flp, act<<lacZ flies were cultured at 18°C until 13–20 days of adulthood, some background activation was detected, as scattered, individual LacZ+ cells (Figure 2A,A’) plus a field of cells in the posterior R5 region, abutting the pylorus (Figure S3A). However, after 2 days of incubation at 29°C (6–10 days old flies at dissection), the pylorus was marked with LacZ expression (Figure S3B–C), as expected, and the midgut epithelium showed more, sparse LacZ+ cells, either isolated or in pairs (Figure 2B,B’). When cultured for 8 days at 29°C (11–18 days old flies at dissection), more cells expressed lacZ in a salt and pepper pattern, with bigger groups that included polyploid ECs as well as diploid cells (Figure 2C,C’). These observations are likely the result of additional clonal induction accompanied by clonal expansion of previously labelled ISC, rather than of wg being expressed coordinately by patches of cells of multiple differentiated cell types. For the flies cultured for 8 days at 29°C, we also inspected the muscle layer of the entire posterior midgut, which showed no wg expression from the muscle cells (Figure 3). Taken together, our results indicate that under homeostatic conditions, wg is expressed intermittently and asynchronously in the Drosophila midgut epithelium, possibly in diploid cells including the ISCs.
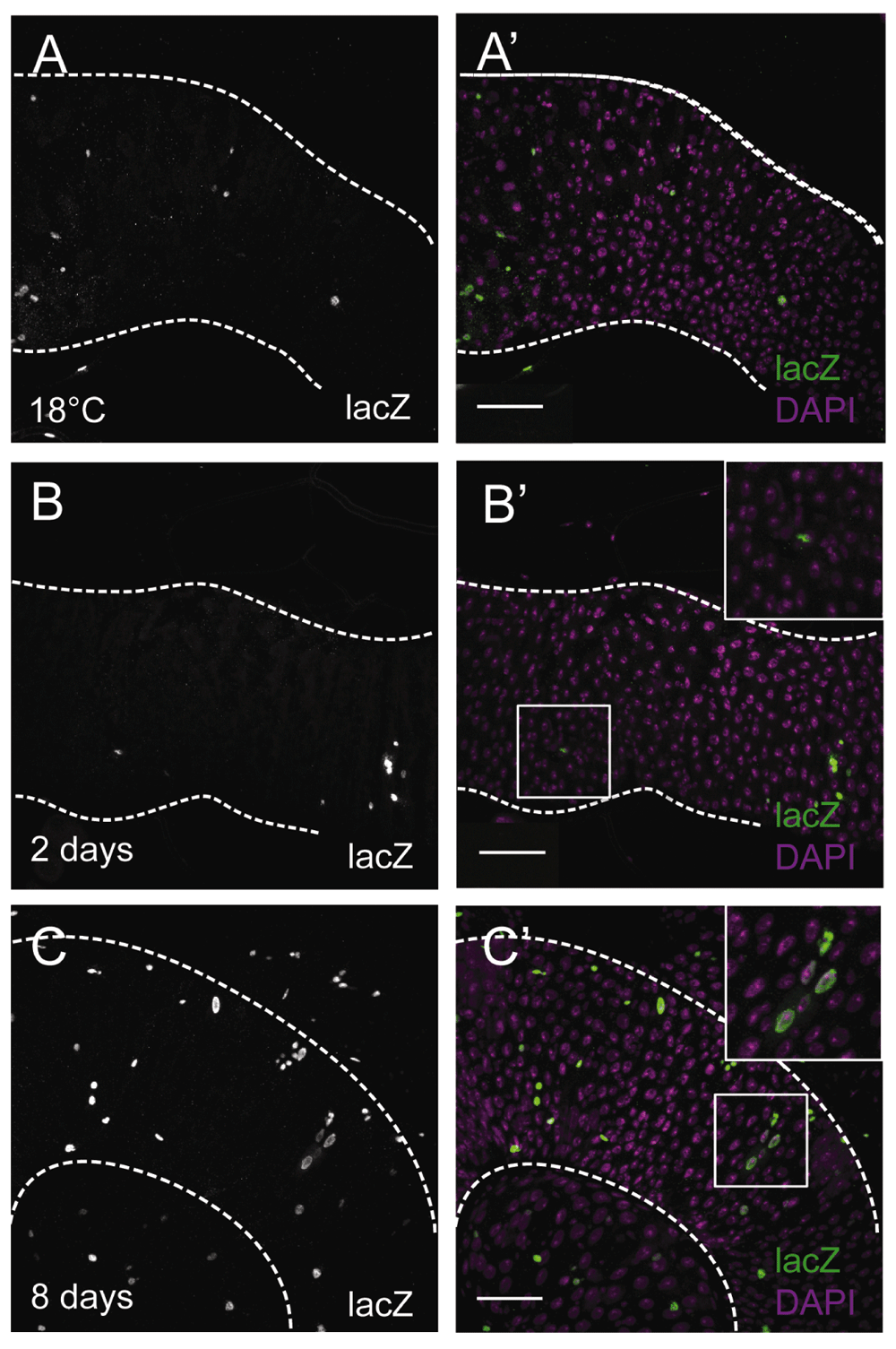
(A,A’) wg[KO]-Gal4, UAS-Flp, tub-Gal80ts, Act<stop<lacZ guts at 18°C (13–20 day old flies). (B–C’) wg[KO]-Gal4, UAS-Flp, tub-Gal80ts, Act<stop<lacZ guts after 2 days (6–10 day-old flies) or 8 days (11–18 day-old flies) of incubation at 29°C for Gal4 activation. (B,B’)Small LacZ+ (anti-βGal) (grey, B; green, B’) clusters of 1~2 cells, mostly diploid, after 2 days at 29°C. (C,C’) Larger LacZ+ (anti-βGal) (grey, C; green, C’) clusters of both diploid and polyploid cells after 8 days at 29°C. DAPI nuclear staining is shown in magenta. Inset boxes show selected regions at higher magnification. Scale bars: 50 μm. Fields of view correspond to the anterior R5 region.
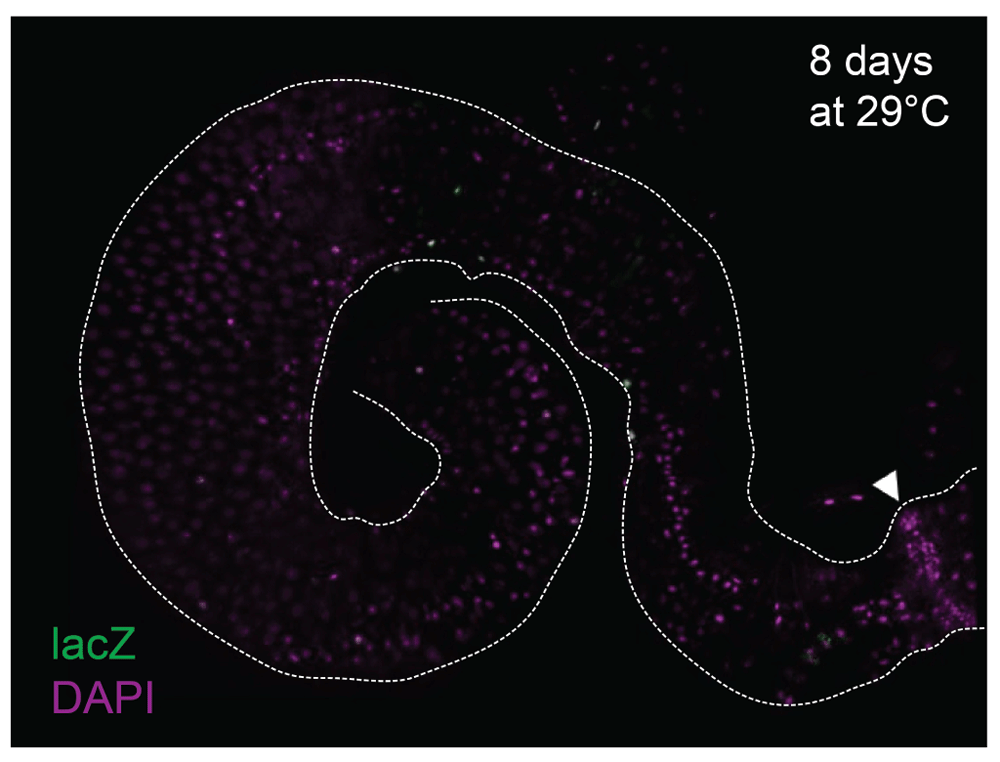
The muscle layer of the wg[KO]-Gal4, UAS-Flp, tub-Gal80ts, Act<stop<lacZ gut after 8 days of incubation at 29°C for signal induction. No LacZ signals (anti-βGal, green) are detected in the muscle cells. DAPI nuclear staining is shown in magenta. Arrowhead points to the pylorus. Scale bar: 50 μm.
In order to further characterise the spatial organisation of the Wg signalling pathway in the Drosophila midgut, we observed the expression patterns of the receptor frizzled2 and the component of the destruction complex shaggy (sgg). Using a frizzled-2 enhancer trap insertion (fz2-GFP), we found that fz2-GFP was highly expressed at the pylorus (Figure 4A,A’). fz2-GFP could also be observed in diploid cells (presumably ISCs and EBs) and EEs away from the pylorus (Figure 4A’). There was no fz2-GFP expression in ECs.
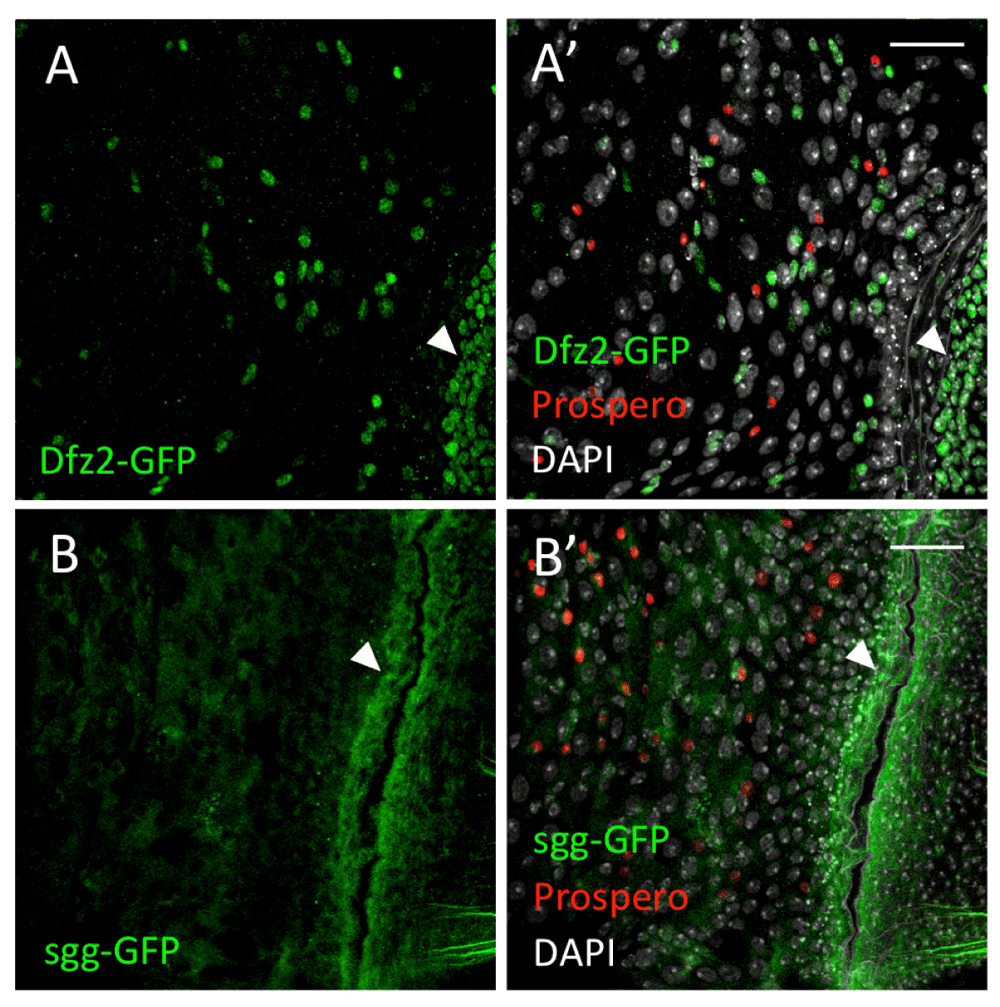
(A,A’) fz2-GFP (green) is expressed in the small cells and the pylorus (arrowheads). (B,B’) sgg-GFP (green) is expressed in all cell types, with strong signals at the pylorus (arrowheads). Prospero (red) marks the EEs. DAPI nuclear staining is shown in grey. Scale bars: 25 μm.
To examine the expression pattern of sgg, we used a Sgg:GFP protein trap. Sgg:GFP could be detected throughout the posterior midgut, in all cell types, with strong expression at the pylorus (Figure 4B,B’).
Next we wanted to examine the activation of Wg signalling in the adult Drosophila midgut. We used a frizzled-3 (fz3) reporter to monitor Wg signalling activity, fz3-RFP, since fz3 is a direct target of the Wg pathway (Olson et al., 2011). We inspected flies expressing fz3-RFP and wg-Gal4 > UAS-GFP, which both showed high expression at the pylorus (Figure 5A,C–C’), with fz3-RFP exhibiting a signalling gradient, culminating at the pylorus (Figure 5C). fz3-RFP also displayed strong epithelial expression in the region abutting the gastric zone (Figure 5A,C’). To identify the cell types expressing fz3-RFP, we used esg-lacZ to label ISCs/EBs, and GBE-Su(H)-GFP to distinguish EBs. We observed that in the regions of the gut away from the pylorus, fz3-RFP is only expressed in ISCs and EBs (Figure 6). However, the stability of RFP in this tissue is unknown, presumably long, and therefore fz3-RFP expression could be indicative of long-past Wg pathway activation.
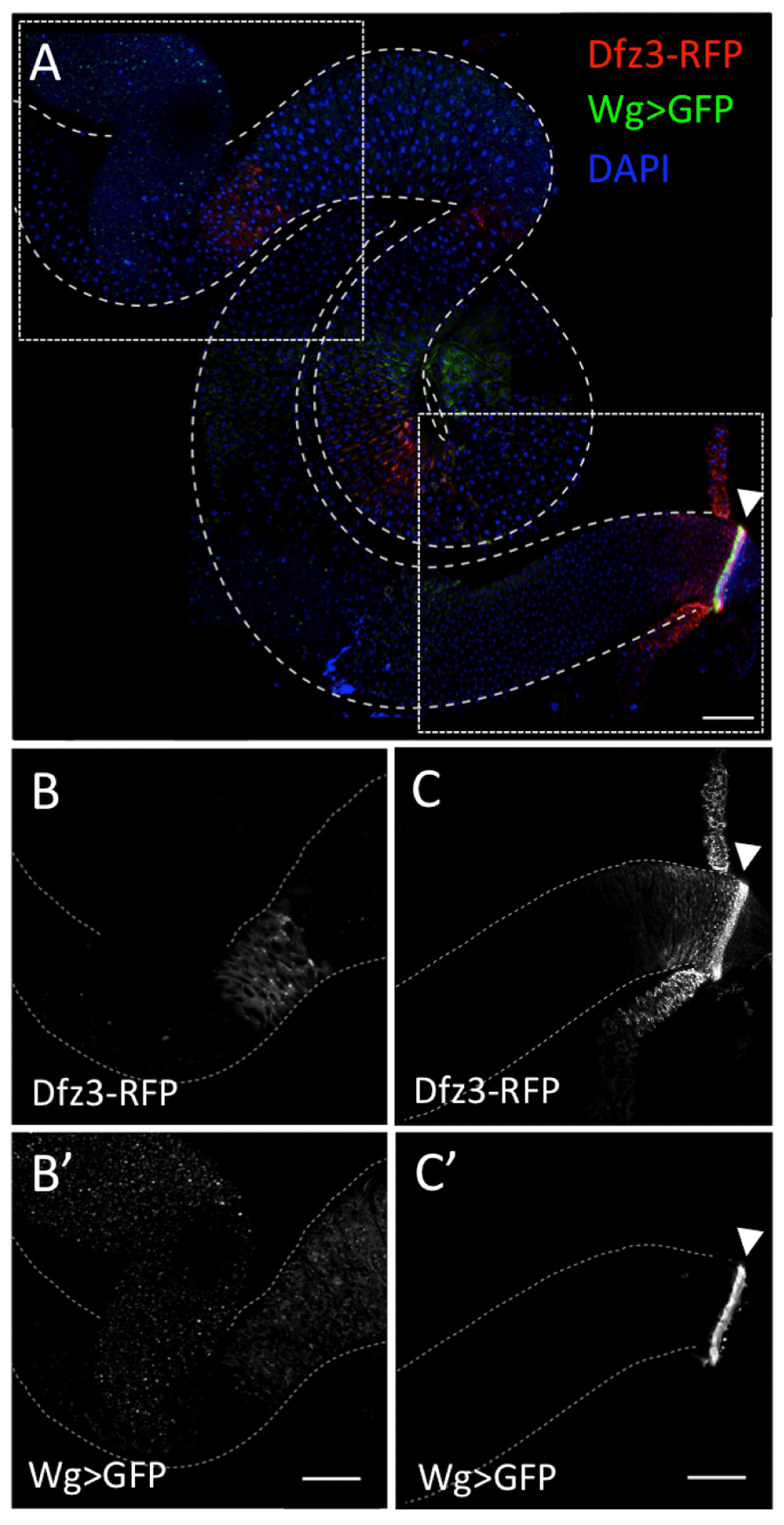
fz3-RFP (red, A; grey, C) and wg-Gal4>UAS-GFP (green, A; grey, C’) are both highly expressed at the pylorus (arrowheads). fz3-RFP also displays epithelial expression in the region abutting the gastric zone (red, A; grey, B). (B,B’) and (C,C’) each show the region of the midgut within the dashed box above. Scale bars: 100 μm.
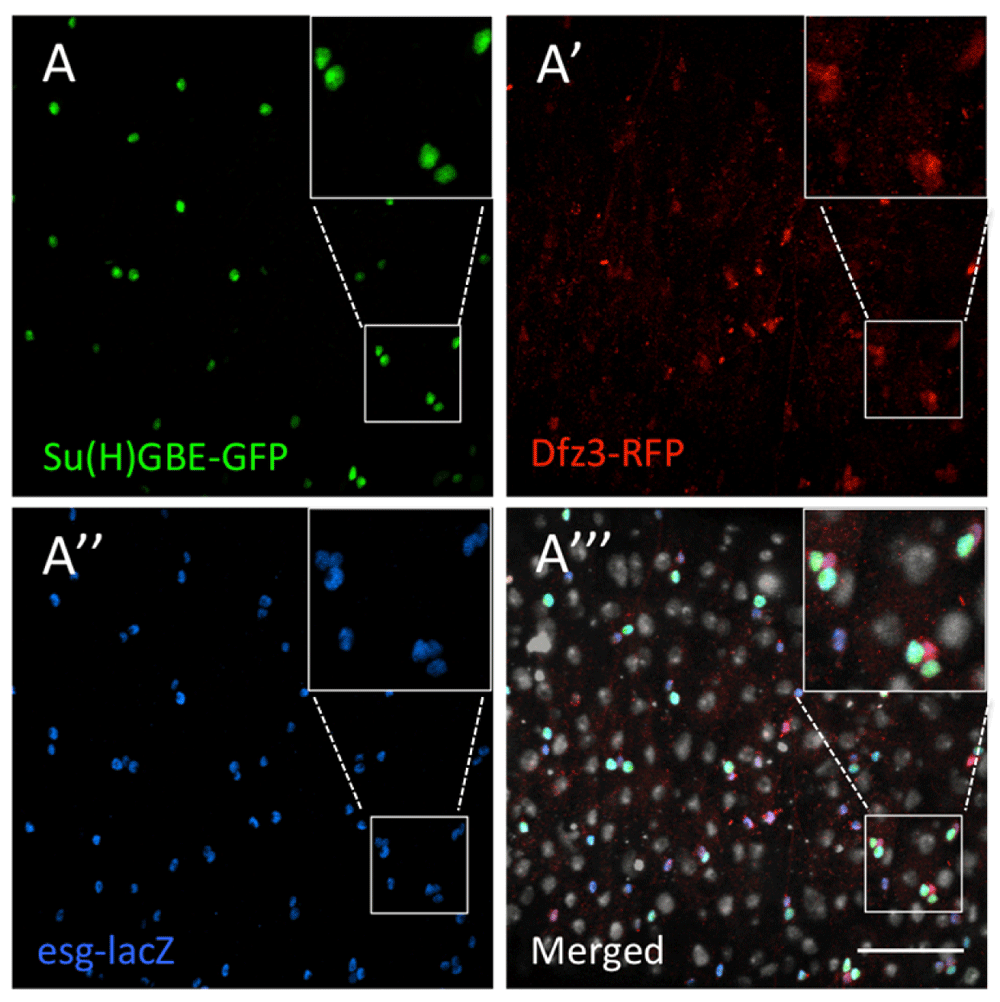
(A) Su(H)GBE-GFP (green) labels EBs. (A’) Fz3-RFP (red) marks the cells responding to Wg signalling. (A’’) Esg-lacZ (anti-βGal, blue) labels ISCs and EBs. (A’’’) Merged. Fz3-RFP (red) is detected only in the ISCs and EBs, and all the ISCs and EBs showed fz3-RFP expression. DAPI nuclear staining is shown in grey. Inset boxes show selected regions at higher magnification. Scale bar: 50 μm.
A more instantaneous reporter for Wnt signalling is the cytosolic levels of the overexpressed nuclear effector of the pathway, Armadillo/β-catenin (Alexandre et al., 2015; Hayward et al., 2005; Lin et al., 2008). When full-length armadillo (armFL) is overexpressed in the epithelium of the imaginal wing disc, Armadillo protein is only accumulated close to the domains of wg expression (Hayward et al., 2005). Arm is so rapidly degraded that its overexpression is only detected in cells where Wg signalling stabilises the protein. Therefore, the accumulation of UAS-ArmFL correlates with presently active Wg signalling. We tested whether ISCs/EBs showed stabilisation of UAS-armFL using esg-Gal4, UAS-GFP. Surprisingly, only a proportion of the GFP-expressing ISCs and EBs showed elevated levels of Arm (Arm+GFP+/GFP+ per field of view: average% = 29%, highest% = 52%, lowest% = 19%) (Figure 7 and Table S1). This suggests that Wg signalling is activated in selected ISCs and EBs only in short periods of time, without any obvious spatial pattern. This is in good agreement with our observations of wg expression, especially as reported with the “tracer amplifier”, wg{KO}ts>Flp, act<<lacZ. The results indicate that Wg production from the ISCs and EBs occurs randomly, eliciting a paracrine/autocrine response that is similarly unpatterned.
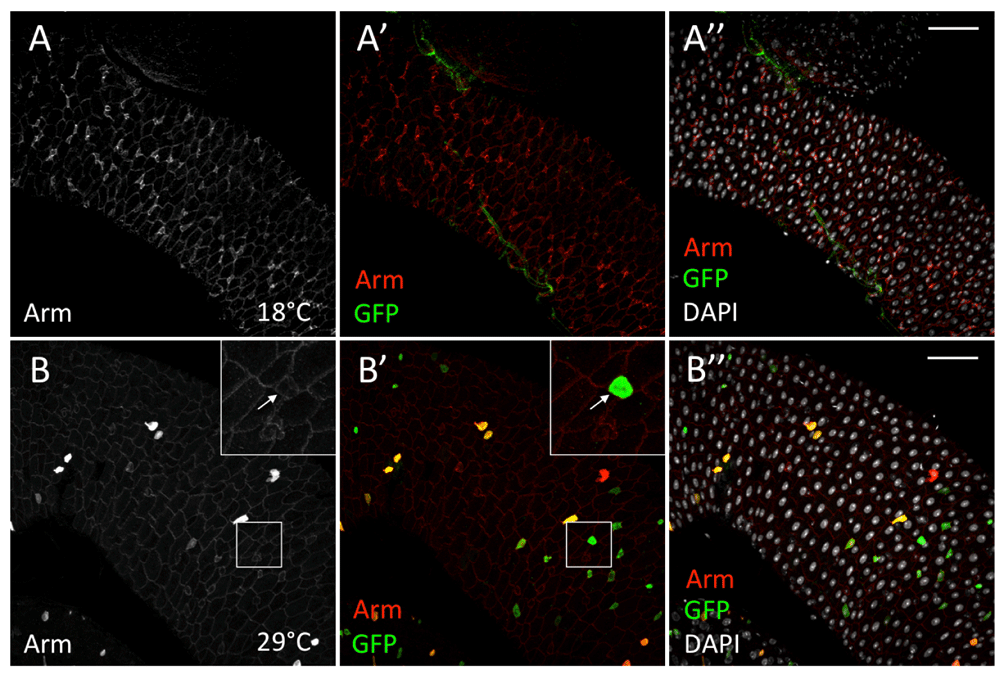
(A–A’’) esg-Gal4[TS], UAS-arm, UAS-GFP intestines at 18°C show no GFP nor ArmFL induction. (B–B’’) GFP (anti-GFP, green) is detected in esg+ cells without ArmFL accumulation (anti-Arm) (grey, B; red, B’) (arrows). DAPI nuclear staining is shown in grey. Inset boxes show selected regions at higher magnification. Scale bars: 50 μm.
We also overexpressed armFL in all cells with the tub<stop<GAL4 driver, which was induced at adulthood by hs-Flp. Only a fraction of the cells, which included both differentiated and undifferentiated cells (ISCs/EBs, marked by anti-HRP), showed high levels of both cytoplasmic and nuclear Arm (Figure 8B–B’’). Arm antibody detected irregular Arm distribution at the cell membranes (Figure 8B–B’’). By contrast, uninduced tissue showed regular wild-type Arm staining at the cell membranes, with higher levels in ISCs and EBs (Figure 8A–A’’). Global arm overexpression suggests that cells in the midgut tissue respond to Wg signalling asynchronously. Also, this forced global Arm induction appeared to perturb regular Arm distribution in the midgut, and possibly disrupting cell packaging, leading to tissue dysplasia. Taken together, the adult Drosophila midgut tissue appears to respond to Wg signalling and stabilise Arm in an unpatterned way.
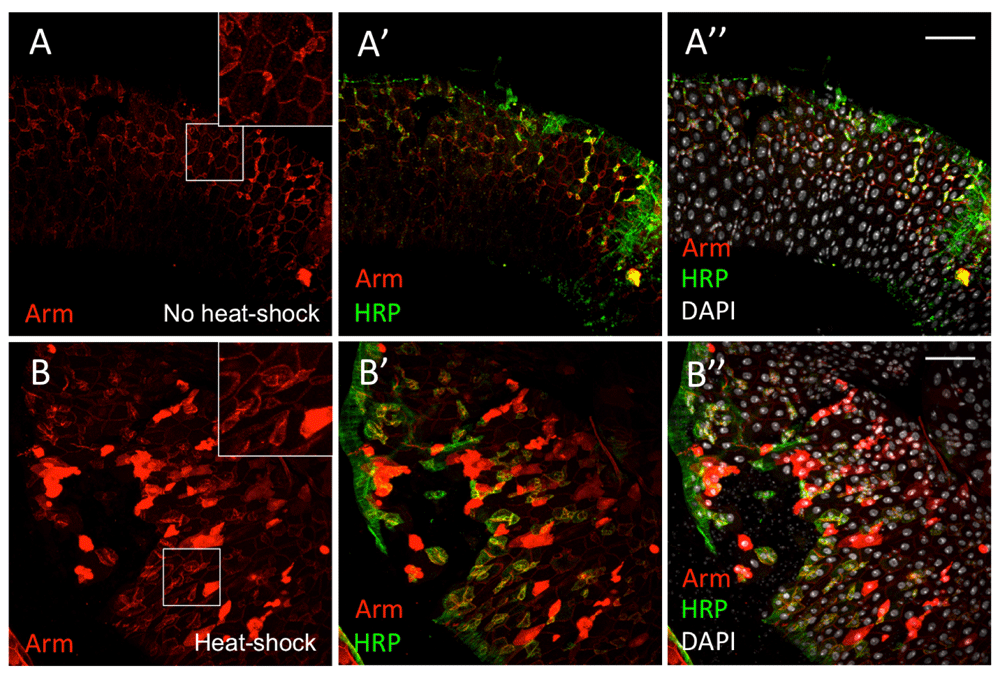
(A–A’’) heat-shock FLP, tub<STOP<Gal4, UAS-arm without heat-shock induction of UAS-ArmFL overexpression. (B–B’’) With the tub<stop<GAL4 driver, UAS-ArmFL is induced in all cells, but only a proportion of the cells shows elevated Arm (anti-Arm, red) signals in the cytoplasm and nucleus. HRP (anti-HRP, green) labels ISCs and EBs. DAPI nuclear staining is shown in grey. Inset boxes show selected regions at higher magnification. Scale bars: 50 μm.
Wnt signalling is the primary driving force in intestinal homeostasis and tumorigenesis in mammals. The adult Drosophila midgut is a powerful system to study intestinal homeostasis, but the role and organisation of Wg signalling in this tissue is still not well understood. We studied the expression, localisation and activity of Wg, and found that in homeostasis, Wg forms a gradient from the pylorus into the posterior midgut, and is also expressed in the diploid cells of the midgut. Moreover, midgut expression appears to be discontinuous and asynchronous, eliciting Wg immediate response in a seemingly random pattern, in turn possibly maintaining long-term expression of Wg signalling reporters.
Previous studies have suggested the visceral muscle as the main production site of Wg in the adult Drosophila midgut, which acts as a stem cell niche (Lin et al., 2008). However, a source of Wg that could comprehensively regulate the widely distributed ISC lineages throughout the midgut is unclear. Using a variety of reagents, we observed a gradient of Wg signalling across the midgut with a source of high wg expression in the epithelium of the pylorus, which agrees with previous reports (Takashima et al., 2008). However, in contrast to Lin et al. (2008) and Tian et al. (2016), we observed minimal levels of wg expression in the muscle cells using different lines and methods. It is possible that the wg expression detected in the muscle is confined to a specific region of the midgut, and that Wg is not being secreted from the entire muscle layer of the midgut, though we have not observed any area of wg expression from the visceral muscles. There might be discrepancies between the distribution of the Wg protein and the wg transcriptional reporters, which might differ in the readouts of the endogenous wg expression. This highlights the significance of the wg{KO}>Flp, act<<lacZ experiment we conducted, in which LacZ could precisely and sensitively reflect the spatial and temporal distribution of Wg. In these experiments, we were able to confirm Wg production from the midgut epithelial cells. Surprisingly, we observed sporadic LacZ+ clones composed of mainly diploid cells after a short gene induction time, while LacZ+ polyploid cells could be detected after longer gene induction. This suggests that wg is only expressed in the diploid cells including ISCs, which later give rise to polyploid ECs.
Using Wg signalling reporters, our work further showed that ISCs and EBs are the cell types responsive to Wg signalling, and that they respond in a stochastic manner. The fz3-RFP reporter seems to reveal a ‘memory’ of past Wg signalling, possibly explaining the results of Tian et al. (2016), where they saw that ISCs and EBs maintain fz3-RFP expression in the absence of Wg signalling. However, Arm stabilisation acts as a readout of the instantaneous response to Wg signalling, and this indicates that ISCs/EBs are indeed responding to Wg signalling. Depending on the stability of the fz3-RFP reporter, it is possible that the midgut epithelium only needs occasional bursts of Wg production to maintain signalling levels. Together with our findings on the sources of Wg production, these results suggest that Wg might act as both paracrine and autocrine signals in the Drosophila midgut, and the two types of signals act in a complementary manner. The paracrine Wg signals would elicit a stronger cellular response in the vicinity of the pylorus, where Wg production is high, and contribute to the spatial patterning of ECs (Buchon et al., 2013; Tian et al., 2016). On the other hand, at the regions further away from the pylorus, where paracrine Wg signalling is weak, ISCs and EBs themselves produce, in asynchronous bursts, the required Wg signals.
Flies were raised and maintained at 18, 25 or 29°C with 65–75% humidity and a 12 hour light/12 hour dark cycle on standard cornmeal/yeast medium (consisting of 1.25% agar, 10.5% dextrose, 10.5% maize, 2.1% killed yeast, and 3.5% nipagin. Supplier: Brian Drewitt, Cambridge, UK) seeded with live yeast. Stocks were obtained from the Bloomington Drosophila Resource Center unless otherwise stated. The NRT-Wg and UAS-GFP:Wg lines were provided by J. Vincent. The fz3-RFP line is from R. DasGupta. Experiments were conducted in well-fed, mated females, 3–20 days old of the following genotypes:
Figure 1:
Oregon R.
+; wg-Gal4/ CyO; UAS-GFP/ TM3, Ser
+/ w; wg-Gal4/ UAS-GFP:Wg; +/ MKRS or TM6B
+; NRT-wg; +
Figure 2,3:
w; wg{KO; Gal4}/ UAS-Flp; tub-Gal80ts/ Act<stop<lacZ
Figure 4:
w; +; fz2CB02997
w, sggCPTI000023; +; +
Figure 5:
w; fz3-RFP/ wg-Gal4; UAS-GFP/ TM6B, Sb, Tb
Figure 6:
y, w; esg-lacZ/ fz3-RFP; Su(H)GBE-GFP/ TM6B
Figure 7:
y, w; esgNP7397/ +; UAS-arm/ tub-Gal80ts, UAS-GFP
Figure 8:
y, w, hs-FLP1.22; tub<GFP, stop<Gal4 / +; UAS-arm / +
Figure S1:
+; wg02657 cn1/ CyO; ry506
The following fixation methods were used: (1) Adult intestines were dissected and collected in BBS for up to 30 minutes, then fixed for 2 hours at room temperature in 4% PFA diluted in BBS. This method was used for most of the experiments. (2) Adult intestines were dissected in ice-cold “wash solution” (ddH2O + 0.7% NaCl + 0.05% Triton) for up to 15 minutes and collected within a mesh basket. The basket was submerged in a “double beaker” with wash solution at 90°C for 5 seconds, and then immediately placed in ice-cold wash solution for 2 minutes. The double beaker was prepared placing a 250 ml beaker inside a 600 ml beaker, both containing wash solution and set on a hotplate until the temperature in the 250 ml beaker reached 90°C (about 30–45 minutes). This method was used for stainings of Armadillo.
After fixation, the tissue was rinsed three times in PBT (PBS containing 0.1% Triton X-100), then washed three times with blocking buffer (PBT containing 2% BSA and 2% FCS), each time 15 minutes on the rotator at room temperature. Primary antibody incubations were overnight at 4°C. After washing with PBT (3 × 15 minutes), secondary antibodies were incubated for 2–4 hours rotating in the dark at room temperature. DAPI (1 μg/ml) was added after the final wash.
Primary antibodies were mouse monoclonal anti-Wg (1:100, gift from J. Vincent) (Alexandre et al., 2015), goat polyclonal anti-HRP (1:500, Jackson, code number 123-001-021) (Hönigsmann et al., 1975), rabbit polyclonal anti-βGal (1:10,000, Cappel) (de Navascués et al., 2012), mouse monoclonal anti-Pros (1:200, Developmental Studies Hybridoma Bank) (de Navascués et al., 2012), mouse monoclonal anti-N27 Arm (1:20, made in the Martinez-Arias lab), chicken polyclonal anti-GFP (1:200, Abcam, ab13970) (de Navascués et al., 2012). Alexa fluorophor-conjugated secondary antibodies (1:500) were from Invitrogen: anti-mouse 568 (Catalog #A-11004, A10037), anti-rabbit 488 (Catalog #A-11034, R37118), anti-rabbit 633 (Catalog #A-21071), anti-goat A488 (Catalog #A-11055), anti-goat A633 (Catalog #A-21082), anti-chicken A488 (Catalog #A-11039). DNA dye was DAPI (Invitrogen).
Tissues were mounted in Vectashield and imaged on Zeiss LSM 700 confocal system using 40× objective and numerical aperture of 1.2.
To induce gene expression, the temporal and regional gene expression targeting (TARGET) method was used with the GAL4, UAS and GAL80ts elements (McGuire et al., 2004). The flies were crossed at the restrictive temperature (18°C), and then the progeny of the desired genotype was allowed to age at 18°C for 3 to 20 days post-eclosion to reach homeostatic condition. The flies were then incubated at 29°C to allow GAL4 activity, inducing the transcription of the UAS transgenes.
The heat-shock flip out system was also used to induce transgene expression (Gordon & Scott, 2009). Adult flies were raised at 25°C until they were 3–20 days old, then they were treated with heat-shock for 30 minutes in a 37°C water-bath. The hs-FLP recombinase was activated upon heat treatment, eliminating the GFP gene and the stop cassette. This activates tub-GAL4, leading to the stimulation of UAS-regulated genes.
Images and figures were assembled using ImageJ (1.47v). Images are maximum intensity projections or selected, representative layers of confocal stacks. The intensity values of wg-Gal4, UAS-GFP:wg were obtained by ImageJ (1.47v) (Figure 1C’), then plotted using RStudio (0.99.491) and Adobe Illustrator CS6 (Figure 1D and Figure S2). Cell counts were conducted using the PointPicker plugin in ImageJ (1.47v) (Table S1). The cellular percentages were calculated using Microsoft Excel 2011 (Table S1).
F1000Research: Dataset 1. Raw microscopy images, 10.5256/f1000research.8170.d115432 (Fang et al., 2016a).
F1000Research: Dataset 2. Raw data for the intensity values of wg-Gal4, UAS-GFP:wg (as plotted in Supplementary Figure S2), 10.5256/f1000research.8170.d115434 (Fang et al., 2016b).
AMA and JdN conceived the project. HYF performed the experiments and collected the data. HYF and JdN analysed the results and wrote the manuscript. AMA discussed the results and commented on the manuscript.
Work by AMA, HYF and JdN was partially supported by the Wellcome Trust. HYF acknowledges the Krishnan-Ang Studentship provided by Trinity College, University of Cambridge.
We thank Jean-Paul Vincent, Ramanuj DasGupta, the Bloomington Drosophila Resource Center, and the Kyoto Drosophila Resource Center for fly stocks, and the Developmental Studies Hybridoma Bank and Jean-Paul Vincent for antibodies.
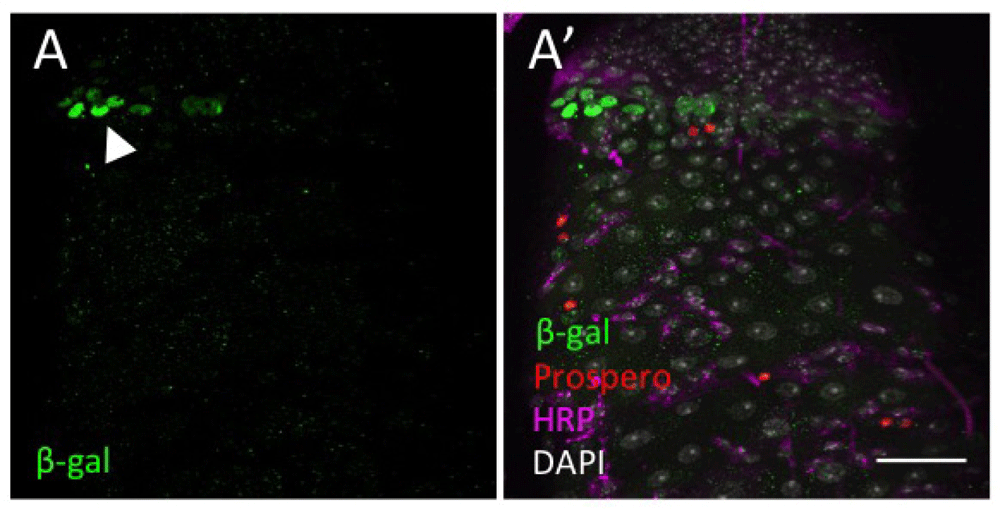
(A) Nuclear β-galactosidase (green) is detected at the pylorus (arrowhead). (B) Prospero (red) identifies the EEs. HRP (magenta) marks ISCs and EBs. DAPI nuclear staining is shown in grey. Scale bar: 30 μm.
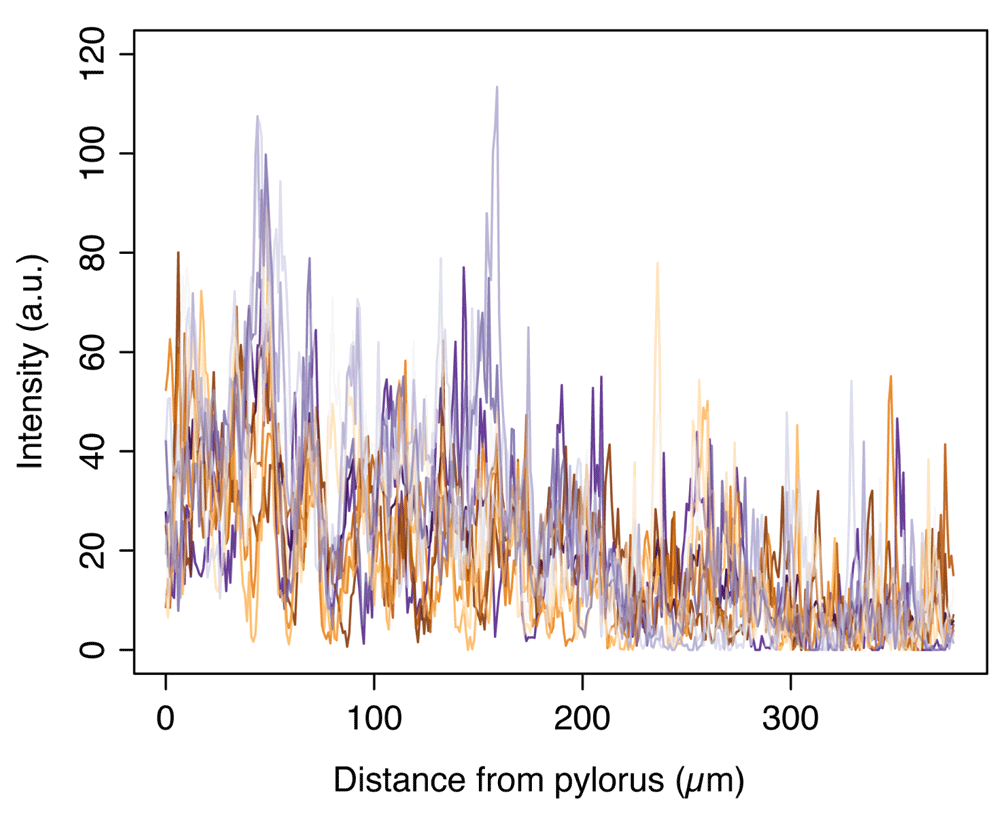
Intensity values of wg-Gal4, UAS-GFP:wg (Figure 1C–C’) along thirteen parallel lines from the pylorus into the posterior midgut (example shown as arrow in Figure 1C’), of which the smoothened average is shown in Figure 1D.
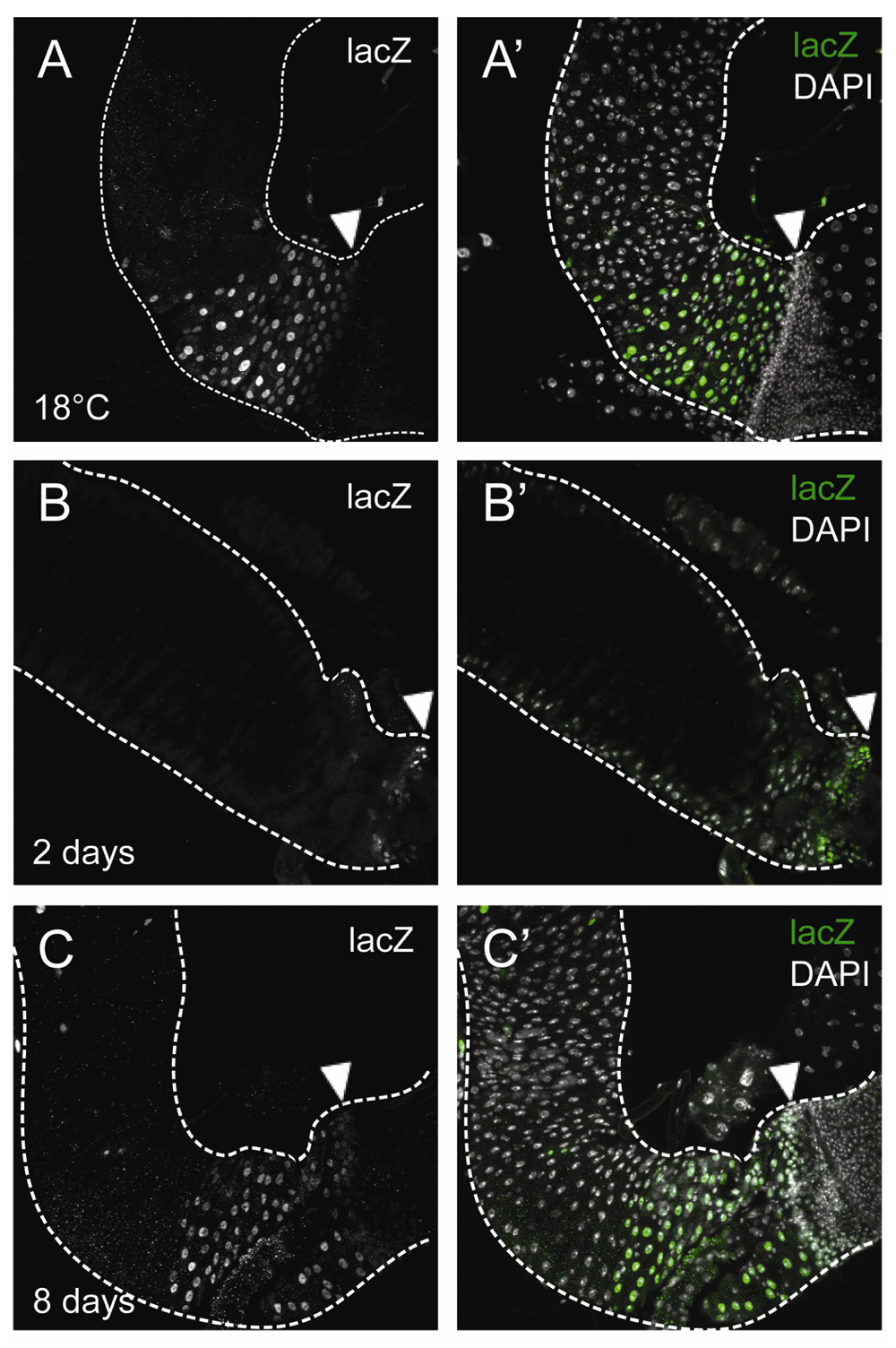
(A,A’) wg[KO]-Gal4, UAS-Flp, tub-Gal80ts, Act<stop<lacZ guts at 18°C (13–20 day-old flies). Note the non-specific induction at the midgut region connecting with the pylorus. (B–C’) wg[KO]-Gal4, UAS-Flp, tub-Gal80ts, Act<stop<lacZ clones after 2 (6–10 day-old flies; B,B’) or 8 days (11–18 day-old flies; C,C’) of incubation at 29°C for clonal induction. LacZ+ cells (anti-βGal, green) are detected in the pylorus (arrowheads) after induction at 29°C (B–C’), but not at 18°C (A,A’). Scale bars: 50 μm.
The percentage of esg+ cells overexpressing armFL is calculated for 5 guts (G1–G5), each with 3 fields of view (F1–F3), from the pylorus into the posterior midgut.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
References
1. Lin G, Xu N, Xi R: Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells.Nature. 2008; 455 (7216): 1119-23 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
References
1. Tian A, Benchabane H, Wang Z, Ahmed Y: Regulation of Stem Cell Proliferation and Cell Fate Specification by Wingless/Wnt Signaling Gradients Enriched at Adult Intestinal Compartment Boundaries.PLoS Genet. 2016; 12 (2): e1005822 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 10 Mar 16 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)