Keywords
larval sampling, Anopheles, Culex, sweep net, dipping
larval sampling, Anopheles, Culex, sweep net, dipping
Traditionally, the larval dip method, as described in detail below, has been the standard method for the collection and sampling of mosquito larvae (O’Malley, 1995). However, this method of collection has proven unsatisfactory for the collection of large numbers of Anopheles mosquito larvae from large water bodies needed for our mosquito genetics studies. Many species of Anopheles, such as An. funestus (Tuno et al., 2007) and An. coluzzii (Gimonneau et al., 2015), readily dive making them difficult to collect from large bodies of water. Collecting Anopheles larvae therefore often means spending considerable time in the field. Also, the larval dip method requires significant experience in dipping techniques and source analysis skills in order to successfully collect the desired genus of larvae (O’Malley, 1995), presenting challenges for a novice.
This limitation of the dipping method motivated us to evaluate a sweep net system similar to methods used by Trapido & Aitken (1953) and Robert et al. (2002) as an alternative approach for collecting larvae to increase catch numbers, especially of Anopheles, and reduce time spent collecting in the field. The method also had to be simple enough for a novice to successfully use. We named our modified sweep net approach the “sweep net catch (SNC)” method and tested it as described below.
A tray 5.7 cm deep × 45.7 cm in length × 31.8 cm in width (BioQuip®Products, Rancho Dominguez, CA- catalogue number 1426c) or a tray similar in size was pre-filled halfway with the cleanest water available from the collecting site and set aside. The sweep net was essentially designed as a sieve consisting of a 25 cm diameter metal ring mounted to the end of a 1.2 meter pole. White nylon or cotton fabric of fine mesh (177.8 × 177.8 mesh per cm or less) was sewn onto the metal ring so water could be sieved through the net. The net was held at a 45° angle and pushed through the water ahead or next to the collector to avoid casting a shadow on the water surface. The top half of the net was above the water surface and the bottom half below and we walked at a slow pace. A visual representation of this process can be seen in the first half of the accompanying Video (time point 0:04 – 1:20 minutes). This process was continued for ten minutes per trial. During the ten minute period, the net was periodically inverted and its contents transferred to the tray containing pre-filled water. After completing ten minutes of sweeping, any mosquito larvae present in the plastic tray were picked out of the tray using a pipette (BioQuip®Products, Rancho Dominguez, CA- catalogue number 4776) and set aside for further cleaning and storage.
A 350 mL dipper (BioQuip®Products, Rancho Dominguez, CA- catalogue number 1132) attached to the end of an approximately 1.2 meter-long pole was used to scoop samples from the water. The collector then inspected the cup for the presence of mosquito larvae. If no larvae were present, the cup was emptied and the collector would try again in another nearby spot. If larvae were present, they were removed using a small pipette (BioQuip®Products, Rancho Dominguez, CA- catalogue number 4776) and transferred to another holding cup prior to taking another dip sample. The collector would continue this process for ten minutes. A visual representation of this process can be seen in the second half of the accompanying Video (time point 1:21 – 2:23 minutes).
Larval collections took place in pools along the Shingwedzi River (23.11604°S; 31.37524°E) and at Lake Panic in Skukuza (24.98472°S; 31.5797°E) in the Kruger National Park, South Africa, and along the shores of a lake in the Lapalala Wilderness area (23.90125°S; 28.29387°E) in the Limpopo Province, South Africa. Five replicate trials were performed along the Shingwedzi River, four trials in Skukuza, and six trials in Lapalala. For each trial the dip method was performed by one collector and the SNC method was performed by another, resulting in fifteen replicates of each method. Collectors, ranging in experience level, alternated collection methods they performed between sites.
All larvae collected were separated by genus and counted. The method used for collection (dip method or SNC method) as well as the collector’s name and experience level (experienced – having dipped for larvae before or novice – having never dipped for larvae before) were also recorded. Cornel and Braack were considered experienced collectors and all others were novices who had never dipped for larvae before. The raw data used for data analysis is available (see Data availability).
A relative abundance of mosquito specimens grouped by genus per collection was calculated. The Wilcoxon-Rank-Sum test implemented in the R statistical package version 3.0.0 was used to compare the efficacy of the two collection methods (Figure 1) and to see if experience level of collectors was a contributing factor in the relative proportions of each genus collected using each collection method (Figure 2).
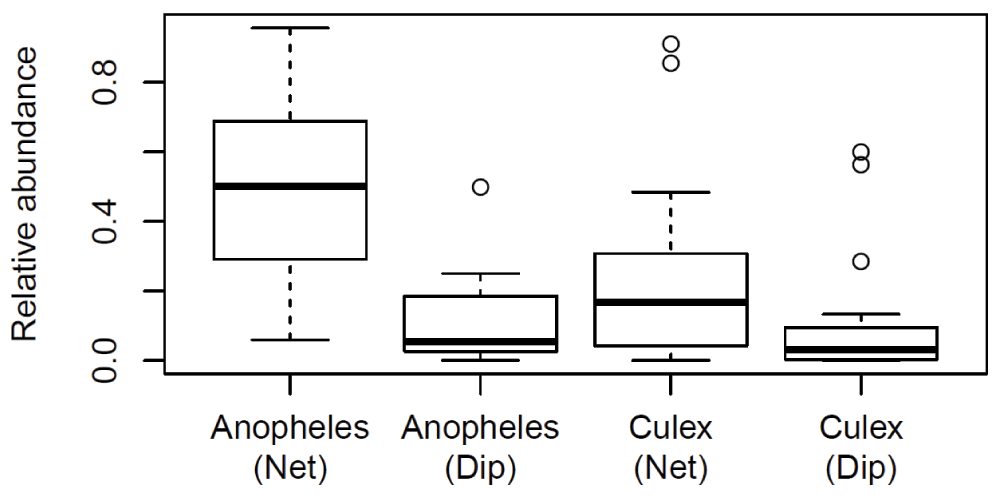
Each collection constitutes a ten minute long sweep net and a ten minute long dip.
As shown in Table 1, a total of 99 Anopheles larvae were collected using the dip method, of which 77 were from Shingwedzi, 4 from Skukuza, and 18 from Lapalala. A total of 605 Anopheles larvae were collected using the SNC method, of which 530 were from Shingwedzi, 20 from Skukuza, and 55 from Lapalala. A total of 61 Culex larvae were collected using the dip method, 22 being from Shingwedzi, 8 from Skukuza, and 31 from Lapalala. A total of 176 Culex larvae were collected using the SNC method, 63 of these from Shingwedzi, 85 from Skukuza, and 28 from Lapalala.
| Anopheles | Culex | |||
|---|---|---|---|---|
| Location | Dip method | Sweep net method | Dip method | Sweep net method |
| Shingwedzi | 77 | 530 | 22 | 63 |
| Skukuza | 4 | 20 | 8 | 85 |
| Lapalala | 18 | 55 | 31 | 28 |
| 99 | 605 | 61 | 176 | |
The number of collected larvae were highly variable between trials, reflecting the general larval density variation between sites. To control for this site variation and compare the relative performance of each collection method, a relative proportion of each genus collected per trial was calculated. The Wilcoxon-Rank-Sum tests on the relative abundance data showed (Figure 1) the SNC method (mean relative abundance = 0.51 ± 0.28) performed significantly better than the dip method (mean relative abundance = 0.12 ± 0.13) in the collection of Anopheles larvae (Wilcoxon-Rank-Sum test P = 8.58 × 10-5). There was no significant difference in the collection of Culex larvae between the SNC method (mean relative abundance = 0.25 ± 0.29) and the dip method (mean relative abundance = 0.12 ± 0.20) (Wilcoxon-Rank-Sum test P = 0.050).
The collector’s experience was not a contributing factor in the relative proportions of each genus collected using either the SNC method or the dip method (Wilcoxon-Rank-Sum test P ≥ 0.21) (Figure 2).
Many of the Anopheles larvae collected were reared to adults and identified using a morphological key (Gillies & Coetzee, 1987) and molecular assays (Koekemoer et al., 2002; Lee et al., 2014; Scott et al., 1993) (see Supplementary material for methods). The species collected included An. arabiensis, An. quadriannulatus, An. coustani, An. pretoriensis and An. funestus group (An. parensis, An. rivulorum, An. leesoni and an as yet undetermined species).
The SNC method performed on average five times better than the dip method in the collection of Anopheles larvae. The SNC samples a larger volume of water than the dip method which likely contributes to the higher catches of Anopheles larvae. Many species, such as An. funestus, An. arabiensis (Tuno et al., 2007) and An. coluzzii (Gimonneau et al., 2015), are known to dive and remain in the substrate for long periods of time when there has been a disturbance on the surface or for feeding purposes. The larger net diameter allows the increased capture of these larvae as they begin to submerge. In shallow parts of the water body the SNC also scoops along the substrate and may collect larvae that have dived and rested there. From our observations the SNC method disturbs the surface of the water less than dipping, which possibly reduces diving behavior.
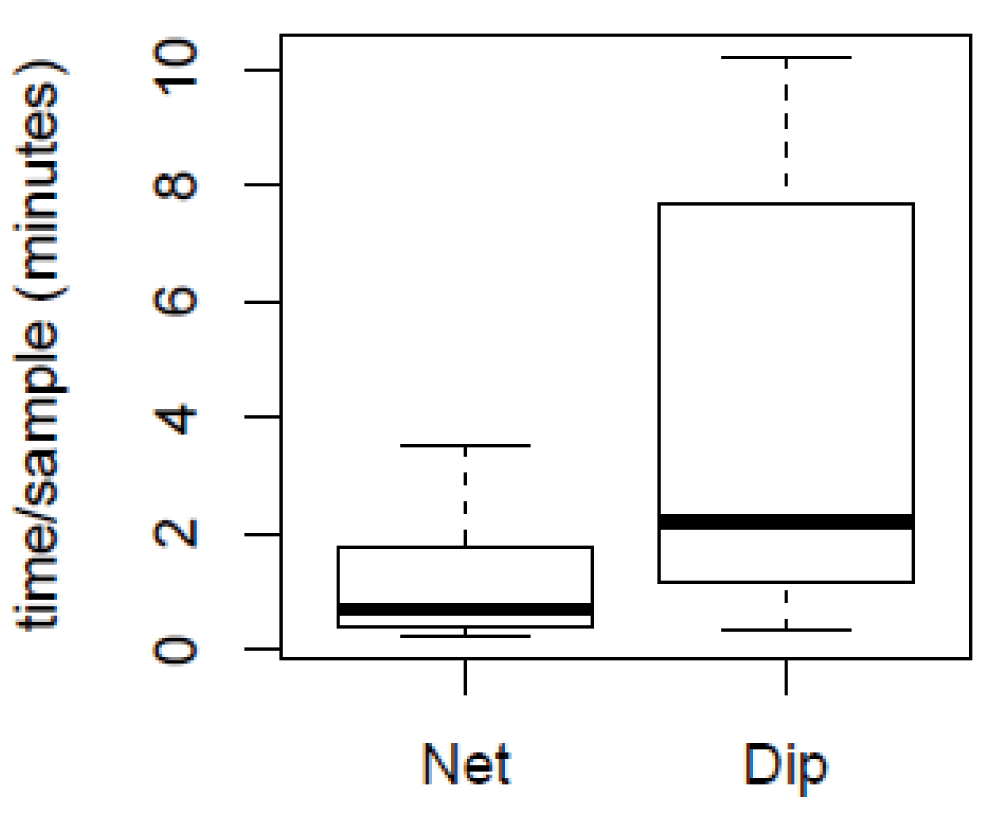
More Anopheles may also have been captured using the SNC because collectors spent more time sieving through the water in the 10 minute period, thus covering a larger area, whereas during the 10 minutes of collecting, the dip method required spending time actively separating out the larvae after each dip. Depending on the water quality and larval density, the final larval separation for the SNC can be a time-consuming task as mud and debris can make it difficult to see the larvae in the tray. However, the overall processing time (collection and sample separation) per specimen for the SNC (1.09 ± 0.97 minutes) was 75% less than the dip method (4.17 ± 3.95 minutes) (Figure 3). To calculate the average time spent collecting larvae with the SNC we added 12 seconds of processing time for each larva collected to the 10 minutes of sweeping for each trial. This time was then divided by the total number of larvae collected in that trial (Data availability). The average time collecting larvae with the dip method was calculated by dividing the 10 minute dipping period by the total number of larvae collected for each trial (Data availability).
Most of the Culex larvae collected were successfully reared to adults and identified using the key in Jupp (1996). The collections consisted of Cx. poicilipes, Cx. simpsoni and Cx. neavei. It has been shown that many species of Culex larvae exhibit significant diving behavior (Workman & Walton, 2003), which suggests the SNC would also have increased efficiency in collecting these larvae as long as the surface was not too disturbed and the collector’s shadow was cast behind them. However, due to the relatively low numbers of Culex larvae collected throughout our study, we suspect there wasn’t a high enough population density of these larvae at any of our trial sites to accurately determine if either method was more efficient for the collection of Culex larvae. We recommend further research be conducted in areas where Culex larvae occur at higher densities to further evaluate whether the SNC performs better than the dip method.
Even though our current data does not show a significant difference in the relative proportion of larvae collected due to the collector’s experience, our personal observations suggest the SNC is a good method for novices to use. The SNC allows the inexperienced handler to easily collect high numbers of mosquito larvae without analyzing their technique or source characteristics as is required to be successful using the dip method (O’Malley, 1995). A larger trial size may illuminate more conclusively if experience level does affect collection performance.
We endorse and encourage the sweep net method as a preferred technique for larval collection that can be easily used in the field setting regardless of experience level. Our SNC method is particularly effective in capturing Anopheles mosquito larvae. The increased sensitivity of SNC towards Anopheles larvae may be due to (1) the sampling of a larger volume of water than the dipping cup, and/or (2) reducing disturbance of the water surface resulting in fewer larval dives. This increased sensitivity of the SNC method makes it an appropriate larval collection tool for studies when more accurate assessments of larval densities are required and when there is less time available to sample for larvae. In addition, the simplicity of the SNC method makes it a recommended choice for novice collectors. Further research is suggested to more rigorously test if a significant correlation between the collector’s experience level and the relative proportion of larvae collected by either method exists.
Figshare: Visual representation of both the sweep net catch and dip larval collection methods as performed by Braack and Cornel. 10.6084/m9.figshare.3123274 (Brisco et al., 2016a).
Figshare: Raw larval collection data for all 15 replicates, including the relative abundance of Anopheles and Culex collected and the estimated time taken to collect one larva for each replicate. 10.6084/m9.figshare.3123463 (Brisco et al., 2016b).
AC and LB conceived the study, designed the experiments, conducted the field work, and filmed the accompanying video. YL performed An. gambiae complex identification assays and data analysis and made figures for the manuscript. KB compiled and edited the accompanying video and made the table for the manuscript. JM performed the An. funestus group molecular assays. All authors were involved in the writing of the manuscript and have agreed to the final content.
Both Cornel and Braack were beneficiaries of a Carnegie African Diaspora Fellowship Program grant (IIE Grantee ID: 15410201) which partly enabled this study.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank Takalani Nelufule (TN) of the Zoonoses Research Unit of the University of Pretoria, as well as the following students of the Lapalala Wilderness School Phumzie Mbonani (PM) and Thoko Jiyane (TJ), and Kruger National Park staff Danny Govender and Purvance Shikwambana for their assistance with the larval collections within Lapalala. Danny Govender also coordinated the field trips in Kruger National Park.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 21 Apr 16 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)