Keywords
Interactome, Proteomics, AP-MS, affinity purification-mass spectrometry, XL-MS, cross-linking MS analysis, PCP, protein correlation profiling, BioID, APEX
Interactome, Proteomics, AP-MS, affinity purification-mass spectrometry, XL-MS, cross-linking MS analysis, PCP, protein correlation profiling, BioID, APEX
A range of complementary approaches are currently being used to identify protein-protein interactions (PPIs) in a large-scale, high-throughput manner (Figure 1). These include affinity purification-mass spectrometry (AP-MS), cross-linking MS (XL-MS) analysis, MS-based protein correlation profiling (PCP), and yeast two-hybrid (Y2H) screens. Proximity labeling techniques, based on the identification (by AP-MS) of near neighbor proteins by spatially restricted enzymes, can also be used to map protein networks and probe complex structures, although they have yet to be applied at a whole proteome level. In this review, we discuss recent applications of these diverse methods to large-scale protein interactome mapping and the public availability of the resulting datasets for both high-throughput bioinformatic analysis of protein interaction networks and single-protein information for more focused studies.
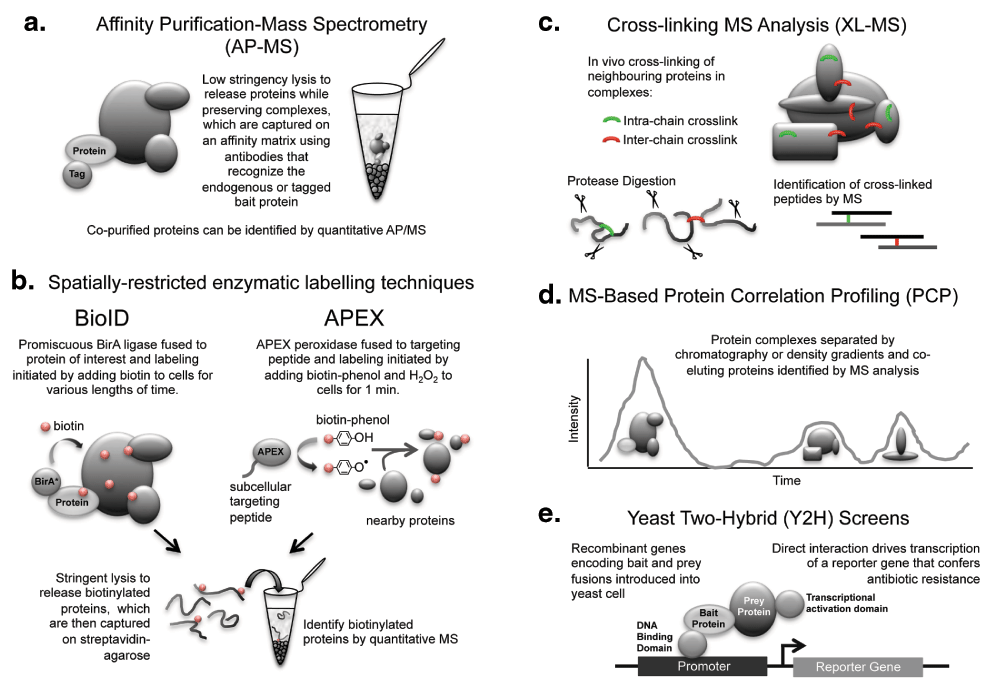
a. Affinity purification-mass spectrometry approach for identifying proteins that associate with a particular bait protein. b. Two spatially-restricted “near neighbor labeling” approaches that utilize enzymatic reactions to tag proteins (for capture and identification) that associate with a bait protein. c. Strategy behind cross-linking mass spectrometry analysis of multiprotein complexes. d. Protein correlation profiling approach for identifying multiprotein complex members that co-elute following various separation techniques. e. Strategy behind the classic yeast two-hybrid method used to screen for direct protein-protein interactions.
Currently, the most popular strategy for both high- and low-throughput interactome mapping is AP-MS, in which an endogenous or tagged bait protein is depleted from cell lysates by using an affinity resin and associated proteins identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Figure 1a). Two recent large-scale studies of human PPIs used AP-MS approaches to identify more than 20,000 interactions, respectively (Table 1). To assemble what they call the BioPlex (biophysical interactions of ORFeome-derived complexes), Huttlin and colleagues C-terminally FLAG-HA tagged about 600 human open reading frames (ORFs) and transiently overexpressed them in HEK293T cells, identifying co-precipitating proteins by AP-MS1. Clone validation, quality control, inclusion of positive and negative controls, and development of a quantitation algorithm (CompPASS-Plus) based on abundance, detection frequency, and reproducibility were employed to increase confidence in the resulting dataset, which was deposited into the BioGRID PPI database last year. The authors consider this to be phase 1 of their long-term effort to map interactomes for the entire human ORFeome collection and are continuing to post updates that can be freely browsed or downloaded from their website.
AP-MS, affinity purification-mass spectrometry; BAC, bacterial artificial chromosome; CORUM, Comprehensive Resource of Mammalian protein complexes; GFP, green fluorescent protein; LC-MS/MS, liquid chromatography-tandem mass spectrometry; MS, mass spectrometry; ORF, open reading frame; XL-MS; cross-linking mass spectrometry; Y2H, yeast two-hybrid.
| Approach | System | Coverage | Dataset Availability | Reference |
|---|---|---|---|---|
| AP-MS experiments identifying proteins that co-precipitate with GFP-tagged bait proteins | N- and C-terminally tagged mouse and human BAC transgenes stably integrated in HeLa cells | 28,500 interactions involving 5,400 proteins | Deposited into IntAct: http://www.ebi.ac.uk/intact and the IMEx consortium: http://www.imexconsortium.org | 2 |
| AP-MS experiments identifying proteins that co-precipitate with FLAG- HA-tagged bait proteins | C-terminally FLAG- HA-tagged ORFs in ORFEOME collection v8.1 transiently overexpressed in HEK293T cells | 23,744 interactions involving 7,668 proteins | Deposited into BioGRID: http://thebiogrid.org Updates can be browsed or downloaded at: http://gygi.med.harvard.edu/projects/bioplex | 1 |
| XL-MS study utilizing MS-cleavable cross-linkers combined with sequential CID-ETD-MS/MS acquisition and XlinkX search engine | HeLa cell lysates | 2,179 unique cross-links detected (1,665 intraprotein and 514 intraprotein) | Reported in Supplementary Data and raw files available as project #890 here: https://chorusproject.org XlinkX publically available: http://sourceforgenet/project/xlinkx/ | 38 |
| Yeast 2-hybrid screens | >15,000 human ORFs from hORFeome v5.1 | ~14,000 high-quality human binary protein-protein interactions | Data (published and updated) can be browsed at: http://interactome.dfci.harvard.edu | 45 |
| Native size-exclusion chromatography combined with LC-MS/MS | U2OS cell lysates | >8,000 proteins identified and 1,061 of 1,970 CORUM complexes mapped | Data available at: www.peptracker.com/encyclopediaInformation/ | 41 |
| Biochemical fractionation combined with quantitative MS profiling | HeLa S3 and HEK293 cell lysates | 5,584 proteins identified and 622 putative protein complexes described | Data deposited into BioGRID: http://thebiogrid.org and publicly accessible here: http://human.med.utoronto.ca | 42 |
| Size-exclusion chromatography and MS-based protein correlation profiling | HeLa cell lysates | 7,209 binary interactions clustered into 291 protein complexes | All IDs reported in Supplementary Data and scripts used for analysis available here: http://www.chibi.ubc.ca/faculty/foster/software/ | 43 |
The approach used by Hein and colleagues2 involved screening a library of 1,125 HeLa cell lines with stably incorporated N- and C-terminally tagged mouse and human bacterial artificial chromosome (BAC) transgenes under near endogenous control3 by AP-MS, as demonstrated previously in focused studies analyzing chromosome segregation4 and the function of motor proteins5. In addition to identifying more than 28,000 interactions in their large-scale screen, the authors estimated interaction stoichiometries (based on absolute quantitation of protein abundances in complexes and compared for both N- and C-terminally tagged and mouse and human bait proteins) and measured the relative cellular abundances of interaction partners. An interesting finding was the predominance of weak (i.e., sub-stoichiometric) interactions in the global interactome, which may suggest that stable complexes rely on weak links to connect to each other and to transient/dynamic regulators. The interaction datasets were submitted to both the IntAct database and the IMEx consortium.
Importantly, both studies demonstrated significant overlap with the CORUM (Comprehensive Resource of Mammalian protein complexes) database, a manually curated repository of more than 2,800 mammalian protein complexes6. CORUM is currently considered the “gold standard” PPI database because it is based solely on high-confidence, experimentally verified interactions and does not accept deposition of large-scale datasets (Table 2). Proteome coverage was also high for both studies, as assessed by comparison with datasets generated and shared in recent large-scale whole proteome mapping initiatives (Table 3) such as the MaxQuant Database7–9 (MaxQB), the Human Proteome Map10, and ProteomicsDB11.
| Database | Description | Link |
|---|---|---|
| CORUM | Manually curated repository of experimentally characterized protein complexes high-throughput experiments excluded) |
http://mips.helmholtz-muenchen.de/genre/ proj/corum/ |
| MIntAct | Open-source, open data molecular interaction database (merger of IntAct and MINT databases) curated from literature and from direct date depositions | http://www.ebi.ac.uk/intact |
| The BioGRID Interaction Database | ~750,000 non-redundant interactions drawn from >55,000 publications for 30 model organisms | http://thebiogrid.org |
| IMEx Consortium | Common curation platform for 11 molecular interaction databases | http://www.imexconsortium.org/ |
| Complex Portal | Open-source, manually curated resource to collate protein complexes from >10 major model organisms | http://www.ebi.ac.uk/intact/complex |
| Database | Description | Link |
|---|---|---|
| Human Proteome Map | Proteome data from 30 human tissue samples (17 adult and 7 fetal); 6 purified haematopoietic cells); Proteins encoded by 17,294 genes identified (~84% of total annotated) | http://www.humanproteomemap.org |
| ProteomicsDB | Combined data available from repositories and contributed by colleagues, representing 60 human tissues, 147 cell lines, 13 body fluids; Coverage for 18,097 of 19,629 human genes | https://www.proteomicsdb.org |
| MaxQB | Proteome data from 11 different human cell lines (19,865 total proteins; average 10,361 ± 120 proteins per cell line and other model organisms) | http://maxqb.biochem.mpg.de/mxdb/ |
Although the standard caveats of AP-MS strategies still apply, namely the potential for overexpression or tag-induced artefacts and the predominance of false positives such as non-specific background proteins12–14 and the recently described cryptic protein binding to cloning regions or “scars” where affinity tags are linked to the gene of interest15, these large-scale studies benefit tremendously from the comparison of multiple experiments. Negative controls are largely bait-independent, and thus common contaminants are highlighted by their appearance in numerous unrelated datasets. Moving forward, the limitations of AP-MS can be further minimized by a variety of strategies, including direct affinity tagging of endogenous proteins using the powerful CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/Cas9) gene editing tool16,17, more rigorous assessment of the quality and specificity of antibodies used to capture endogenous proteins for AP-MS18, and improvements in significance analysis software19,20.
Although AP-MS remains the most commonly used technique for mapping PPIs, its Achilles heel has always been the necessity to break cells open to extract complexes for analysis, which can be disruptive to the underlying PPIs and hinder identification of weak or transient associations or both. The development of complementary proximity labeling approaches that use spatially restricted enzymes to biotinylate neighboring proteins has helped to address this key issue. Complex members are labeled covalently in vivo, thus eliminating the need for low-stringency purification strategies to preserve their integrity. Furthermore, the high affinity of streptavidin for biotin facilitates efficient recovery of biotinylated proteins from lysates for MS analysis.
Two particular proximity labeling techniques, BioID and APEX, have been employed recently for the analysis of multiprotein complexes and for identification of the protein components of specific cellular compartments (Figure 1b). BioID involves expression of a protein of interest fused to a prokaryotic biotin ligase and the subsequent biotinylation of amine groups on neighboring proteins when excess biotin is added to the cells. Whereas the wild-type BirA biotin ligase from Escherichia coli is capable of transferring biotin only to a substrate bearing a specific recognition sequence, the generation of a promiscuous BirA (Arg118Gly mutant) permits the biotinylation of any protein found within a 10-nm labeling radius21,22. As with AP-MS, identification of a protein-protein association using BioID does not imply a direct physical interaction.
BioID has enabled the identification of proteins involved in important functional complexes that were previously difficult to characterize because of the limitations of AP-MS. For example, the identification of ubiquitin ligase substrates by AP-MS is challenging and this is due in part to the weak and transient interactions observed between the ligase and its substrates. A BioID approach, however, facilitated identification of novel substrates23. This type of approach has also been used to identify novel c-MYC24 and HIV-1 Gag25 interacting partners, highlight force-dependent molecular interactions at cell-cell adhesions26, identify proteins localized to cell junction complexes27,28 and the centrosome-cilium interface29, and probe the structure of the centrosome30,31 and the nuclear pore complex22.
APEX is a monomeric peroxidase reporter derived from pea32 or soybean33 ascorbate peroxidase that catalyzes the oxidation of biotin-phenol to biotin-phenoxyl in the presence of H2O2, resulting in the biotinylation of proteins in the neighboring region. Whereas BirA-catalyzed biotinylation is limited to Lys residues, biotin-phenoxyl radicals can covalently react with electron-rich amino acids such as Tyr, Trp, His, and Cys. They are also short-lived (<5 ms) and membrane-impermeable and have a small labeling radius (<20 nm). APEX can also catalyze diaminobenzidine precipitation to generate contrast after OsO4 fixation, which allows confirmation of localization at nanometer resolution by electron microscopy32. A second-generation APEX2 (Ala134Pro mutant) with improved efficiency was shown to function even better as both a promiscuous labeling enzyme and an EM tag34. Similar to BioID, once proximity labeling has been achieved, biotinylated proteins can be identified via stringent streptavidin purification and MS analysis. An advantage of APEX over BioID is higher temporal resolution, as labeling is achieved on a minute rather than an hour scale.
The APEX reporter has been used to map the proteome of the human mitochondrial intermembrane space and membrane-enclosed mitochondrial matrix33,35, the Drosophila muscle mitochondrial matrix proteome36, and the proteome of the cilium37. Although the applicability of APEX to interactome mapping out with membrane-bound organelles has not yet been demonstrated, further optimization of the enzyme and substrate could extend its utility.
High-quality large-scale interactome datasets have also been assembled using strategies such as XL-MS, which provides additional information about the topographical structure of protein complexes (Figure 1c and Table 1). In the case of XL-MS, progress was initially slowed by the complexity of data acquisition and analysis, in particular the two overlapping series of fragment ions from each peptide that appear in the MS/MS spectrum. Although major advances have been made38,39, including the development of MS-cleavable cross-linkers that fragment efficiently in the MS/MS mode to yield two major fragment ions corresponding to the component peptides (which can be subsequently identified by MS3), sensitivity can be further improved in the future by the addition of pre-fractionation steps, the use of affinity-tagged cross-linking agents or complementary chemistry (i.e., agents that cross-link amino acids other than lysine40), digestion with complementary proteases, and the development of dedicated software for the analysis of complex XL-MS datasets.
Similarly, PCP-MS studies (Figure 1d) also continue to increase in coverage and specificity, comparing favorably to reference interactome datasets41–43. This approach avoids affinity purification steps and instead separates and maps protein complexes using a variety of approaches that include density gradients and size-exclusion, ion-exclusion, and hydrophobicity interaction chromatography. Given the range of separation options available, PCP-MS also offers significant scope for advancement in the future.
Although XL-MS does identify direct protein interactions, the other approaches discussed above (AP-MS, proximity labeling, and PCP-MS) can confirm only that proteins exist in the same multiprotein complex. A complementary technique that has been used for more than 20 years to detect direct PPI is the Y2H assay. In this approach, the bait and prey proteins are tagged to the DNA binding and transcriptional activation domains of a split transcription factor, and direct binding drives its reconstitution and subsequent activation of a reporter gene (Figure 1e). Although limited by technical and biological challenges that include the need to construct large libraries and the high false-negative and -positive rates that arise from the absence of certain post-translational modifications in yeast that govern protein-protein associations in mammalian cells and forced interactions that do not occur in mammalian cells under physiological conditions, the Y2H screen remains a powerful approach for detecting or confirming (or both) binary interactions.
Using the extensive human ORF collection as bait/prey in an ongoing series of large-scale Y2H screens, researchers at the Dana-Farber Cancer Institute in Boston are addressing the question of which PPIs in the human interactome are direct44,45. With the long-term goal of mapping the full range of human binary PPIs, their most recent update added about 14,000 new binary interactions, bringing the current total to about 17,000. The full dataset, and future updates, can be browsed using their dedicated web interface (Table 1).
With a daunting grand plan for these PPI network maps to comprehensively characterize individual protein functions and global proteome organization, it is not surprising that significant challenges remain. As noted above, the stringency and efficiency of protein extraction and depletion remain an issue with AP-MS studies, and traditional mapping strategies still favor the most abundant/robust interactors. It is hoped that, as complementary approaches such as proximity labeling, XL-MS, and PCP-MS increase in sensitivity and specificity, they will provide extended coverage of the interactome. Importantly, as more high-quality large-scale datasets are collected and shared via online interaction databases like MIntAct46 and BioGRID47 (Table 2), consistencies and patterns will emerge.
Additional technical challenges, posed by their hydrophobic nature, have particularly hampered the identification of PPIs among membrane proteins (and between membrane proteins and soluble proteins such as cytosolic signaling factors). However, the success of recent large-scale initiatives such as the mapping of more than 12,000 binary interactions between Arabidopsis membrane/signaling proteins using the mating-based split ubiquitin system (mbSUS) in yeast48 and the TAP (tandem affinity purification)-MS based screening of 1,590 putative budding yeast membrane proteins using three different mild, non-denaturing detergent purification strategies in parallel49 (1,726 PPIs and 501 putative heteromeric complexes identified) demonstrates that these challenges are also surmountable.
Other challenges include the necessity to define PPIs over a wider range of biological contexts, given that some are cell cycle- or developmental stage-specific, for example, or occur only under particular physiological conditions or in response to specific post-translational modifications. An ambitious future goal is a comprehensive and quantitative high-throughput approach that combines gene-editing with live super-resolution imaging and interactome mapping to define the dynamic localization, composition, and topography of functional multiprotein complexes.
AP-MS, affinity purification-mass spectrometry; CORUM, Comprehensive Resource of Mammalian protein complexes; ORF, open reading frame; PCP, protein correlation profiling; PPI, protein-protein interaction; XL-MS; cross-linking mass spectrometry; Y2H, yeast two-hybrid.
Virja Mehta and Laura Trinkle-Mulcahy wrote the manuscript. Both authors read and approved the final manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 29 Apr 16 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (2)