Keywords
Autologous Cell Therapy, tumor-infiltrating lymphocytes, Immune Checkpoint Inhibitors, DNA-based vaccine, Chimeric antigen receptors,
Autologous Cell Therapy, tumor-infiltrating lymphocytes, Immune Checkpoint Inhibitors, DNA-based vaccine, Chimeric antigen receptors,
Squamous cell cancers of the head and neck, including the oral cavity, pharynx, and larynx, together account for the eighth most common malignancy in the US and represent an even higher percentage worldwide. In 2015, a total of 59,340 new cases and 12,290 estimated deaths were expected1. Despite a decrease in the number of smoking-related cases of head and neck squamous cell cancers (HNSCC), the overall incidence is steady or increased because of the epidemic of squamous cell cancers of the oropharynx attributable to human papillomavirus (HPV).
Although advances in local therapies have led to improved survival for locoregionally advanced HNSCC, fatal local recurrence and metastases remain a very significant problem. Only small advances have been made in survival rates or survival duration with various chemotherapies in the last several decades. Even with modern multi-agent chemotherapy, the expected median survival for a patient with an incurable or metastatic relapse remains under a year, or marginally longer for patients who develop metastases from an HPV-related HNSCC2,3. Improvements in systemic therapy are needed for this group of otherwise incurable patients. In addition, as systemic therapy plays a key role in the multi-modality curative intent therapy for locoregionally advanced HNSCC, better systemic therapy is needed to increase cure rates in patients with locoregionally advanced but not metastatic HNSCC.
Immunotherapy has been tested in the past in HNSCC. Trials with systemic interferon-alpha (IFN-α) or interleukin-2 (IL-2) were disappointing, and response rates ranged from 0 to 6%. One trial examining IFN-α in combination with IL-2 showed only a modest effect; two of 11 patients (18%) exhibited a partial response4. Given the relative lack of success of these trials and others, and the significant toxicity of these drugs, new therapeutic approaches were needed4–10.
Standard-of-care cancer treatment has revolved around three traditional therapies: radiation, chemotherapy, and surgery. With the advent of immunotherapy, the promise of harnessing and focusing the body’s own immune system to combat malignancies has added a powerful weapon to the oncologist’s arsenal. The remainder of this review will focus on currently approved immunotherapy as well as promising immunotherapeutic approaches under clinical investigation.
One of the most heavily investigated areas of cancer immunotherapy has centered on vaccines. They have been applied in most cancer indications and their lack of success has been well documented. However, there continue to be trials with new adjuvants combined with different vaccination components (e.g., peptide, DNA, and bacteria). Within head and neck cancer, a DNA-based vaccine targeting E6 and E7 genes of HPV in combination with IL-12 is currently under investigation in a phase 1 clinical trial (NCT02163057). Recent work with immunomodulatory peptide vaccines for HPV-positive and MAGE-A3-positive tumors elicited antigen-specific T cell and antibody responses to the respective vaccines but ultimately lacked clinical efficacy (NCT00257738)11. Another study incorporated personalized medicine into vaccine therapy in a phase I/II trial investigating the safety profile of a vaccine composed of patient-specific tumor antigen derived from chaperone-rich tumor cell lysate (NCT01998542). A phase I trial was recently completed by using a dendritic cell p53 vaccine, but further studies need to be conducted in order to gauge clinical efficacy12.
Adoptive cell therapy involves the transfer of tumor-reactive T cells, cultured ex vivo, into patients. With improvement in cell culturing (e.g., IL-2 conditioning), these T cells, which traditionally have been isolated from tumors (tumor-infiltrating lymphocytes, or TILs), could be cultured ex vivo, generating a sufficient number of cells before re-introduction into the patient. This therapeutic regimen was pioneered in the field of melanoma and is still employed experimentally for the treatment of various solid tumors, including HNSCC13,14. For HPV-associated HNSCC, a phase 2 clinical trial under way at the National Cancer Institute is generating TILs from metastatic HPV16/HPV18-positive tumors (NCT01585428). Undifferentiated nasopharyngeal cancers have a high association with Epstein-Barr virus (EBV) and can be treated by using EBV-specific T cells. Unlike TIL preparation, EBV-specific T cells can be isolated and expanded from peripheral blood mononuclear cells (PBMCs). Infusion of EBV-specific T cells showed a durable clinical response in four of six patients who had been refractory to chemotherapy and radiation15. Recent results from a phase 2 trial of EBV-specific autologous T cells in combination with gemcitabine and carboplatin showed a 71% response rate (35 patients), but the 3-year survival rate was 37%16. Currently, two clinical trials using autologous EBV-specific T cells are under way: a phase 2 trial using EBV-specific T cells as a monotherapy (NCT00431210) and a separate phase 3 trial using EBV-specific T cells in combination with gemcitabine and carboplatin versus gemcitabine or carboplatin alone (NCT02578641).
In recent years, adoptive cell therapy has incorporated gene transfer techniques to endow T cells with receptors specific for tumor antigens. This allows the use of a naïve (with regard to antigen specificity) T cell population—a population isolated from patients’ peripheral blood rather than from a resected tumor. Chimeric antigen receptors (CARs) are composed of antibody variable domains specific for a cell surface antigen fused to intracellular T cell co-stimulatory and activation domains (4-1BB and CD3 are most commonly used). When introduced into T cells, these chimeric receptors allow the recognition and subsequent activation against a non-human leukocyte antigen (non-HLA) restricted tumor antigen. Recently, much fanfare has been focused on the use of CARs in hematological malignancies because of seminal work by Carl June and colleagues in chronic lymphocytic leukemia17–19. However, the question remains whether the successes in treating hematological cancers can be transferred to solid tumors owing not the least to the suppressive tumor microenvironment against T cells indicative of many solid tumor types (e.g., upregulation of inhibitory receptors on T cells)20. Within HNSCC, there is currently an ongoing phase 1 trial using an ErbB-specific CAR21,22 (NCT01818323). Engineered T cells expressing the T1E28z CAR, an ErbB ligand fused to CD28 and CD3, will be delivered directly by injection into tumors of HNSCC patients with locoregional disease22. Preclinical data of HNSCC CAR-T therapy suggest that this approach could have a therapeutic effect. A CAR recognizing the EBV latent membrane protein 1 (LMP1) exhibited specific cytolytic activity against LMP1-positive nasopharyngeal tumor line and reduced tumor growth in a xenograft model23. A separate study of a CAR targeting chondroitin sulfate proteoglycan 4 (CSPG4) showed reduced tumor growth in a xenograft model of the HNSCC cell line PCI-3024.
Immunological responses are carefully regulated through calculated expression of both stimulatory and inhibitory signals. Immune checkpoint receptors provide an example of inhibitory regulation of adaptive immune responses. Their expression plays an essential role in controlling and reducing tissue damage due to robust immune responses during an infection or as a result of self-directed immunity. Programmed death 1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) are two membrane proteins expressed by T cells that have been identified as key inhibitory surface receptors in T cell function (Figure 1). Their activation is associated with exhaustive phenotypes in tumor-infiltrating CD3+ T cells. Tumors have evolved mechanisms to successfully reduce the anti-tumor response of T cells via induction of such immune checkpoint inhibitors. As such, blockade of these receptors or their ligands is an attractive approach to promote a T cell-directed immune response against the tumor25.
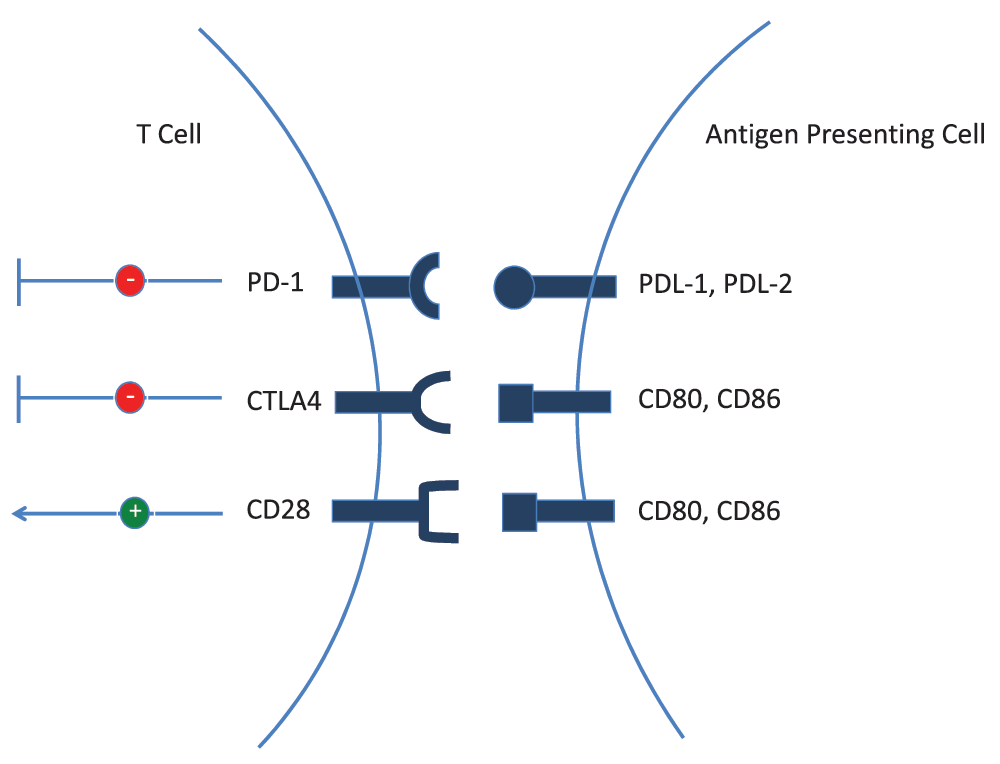
T cell activation is influenced by co-stimulatory signals received via CD28. Conversely, T cell activity can be reduced by upregulation of inhibitory receptors programmed death 1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4).
Nivolumab is an anti-PD-1 monoclonal antibody that was recently approved by the US Food and Drug Administration (FDA) for the treatment of advanced metastatic cancers, specifically melanoma, non-small cell lung cancer, and renal cell carcinoma. A phase 3 clinical trial demonstrated improved overall survival with nivolumab treatment compared with docetaxel chemotherapy in patients with non-small cell lung cancer (39% versus 23% at 18 months)26. In patients with HNSCC refractory to platinum therapy, a phase 3 study of nivolumab monotherapy resulted in improved overall survival compared with treatment with the investigator’s choice of weekly methotrexate, docetaxel, or cetuximab (NCT02105636). Ipilimumab, an anti-CTLA-4 monoclonal antibody, was approved for the treatment of metastatic melanoma in 2011 on the basis of a phase 3 trial that compared it with a gp100 peptide vaccine25. Subsequently, two PD-1 inhibitors, nivolumab and pembrolizumab, were approved for second-line use after ipilimumab. Improved progression-free survival was also observed with nivolumab compared with ipilimumab for metastatic melanoma, both as monotherapy and in combination with an additional checkpoint inhibitor, ipilimumab27. Combined, ipilimumab and nivolumab extended progression-free survival by 8.5 and 4.5 months as compared with monotherapy with ipilimumab or nivolumab, respectively, and the FDA recently approved the combination for melanoma in the first-line setting for BRAF wild-type patients or following BRAF resistance. Most importantly, these drugs appear to lead to “cure” in a substantial portion of patients with metastatic melanoma, though less commonly in other cancers. The amazing success of these checkpoint inhibitors has led to a race for approval in other solid tumor indications, including HNSCC. Focusing on PD-1 blockade, or blockade of its ligand PD-L1 in advanced HNSCC, a multitude of trials are under way (Table 1). Recent studies have concentrated on characterizing PD-1/PD-L1 expression and its correlation with overall outcome. HPV-positive HNSCC tumors were shown to have a unique immune profile compared with HPV-negative tumors. They are associated with a higher density of CD8+ T cells, which can be stimulated to produce IFN-γ and IL-17 in vitro28. Although efforts have focused on the profile of PD-1/PD-L1 intra-tumoral expression, there is a lack of data correlating expression in HPV-positive HNSCC with overall survival28–30. In fact, at least one study unexpectedly correlated improved overall outcomes with increased levels of PD-1+ T cells in HPV-positive tumors31.
It remains to be seen whether adoptive cellular therapy alone will be sufficient to affect a sustainable anti-tumor response in HNSCC. The approvals of checkpoint inhibitor antibodies for melanoma (ipilimumab, nivolumab, and pembrolizumab), non-small cell lung carcinoma (nivolumab and pembrolizumab), and most recently renal cell carcinoma (nivolumab) have paved the way for these foundational therapies; that is, one or more checkpoint inhibitors will likely be incorporated into solid tumor therapeutic regimens. As such, there is an extensive clinical trial program for approval in new indications, including cancers of the head and neck. It is not hard to imagine that the next generation of immunotherapy would combine these checkpoint inhibitors with engineered T cells (e.g., CARs and modified T cell receptors) or even autologous T cells.
However, the attractiveness of antibody therapy is offset by the headache of cellular therapy. Whereas checkpoint inhibitors represent classic “off-the-shelf” biopharmaceutical agents, cellular therapy remains “personalized” medicine. From a manufacturing/business standpoint, it is still not yet feasible to mass-produce cellular therapies, although industry is hard at work solving this problem. As such, adoptive therapy approaches are still years away from “off-the-shelf” status32,33.
BB is a consultant (on the advisory board for melanoma) for Bristol-Meyers Squibb (New York, NY, USA). CSC declares that she has no competing interests.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: Francis Worden is affiliated with clinical trials conducted by the following: Bristol-Myers Squibb, Merck, Eisai, Novartis, Bayer.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 05 May 16 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)