Keywords
Brown adipose tissue, shiver, cutaneous vasoconstriction, thermogenesis, fever, sympathetic nerve activity, preoptic hypothalamus, rostral raphe pallidus, dorsomedial hypothalamus, therapeutic hypothermia, obesity
Brown adipose tissue, shiver, cutaneous vasoconstriction, thermogenesis, fever, sympathetic nerve activity, preoptic hypothalamus, rostral raphe pallidus, dorsomedial hypothalamus, therapeutic hypothermia, obesity
Body temperature (Tcore) is a critical homeostatic parameter influencing cellular function and organismal survival. Life-threatening protein denaturation looms as Tcore increases, and reductions in membrane fluidity, ion fluxes, and enzyme performance accompany significant reductions in Tcore. The fundamental central neural circuits for thermoregulation orchestrate behavioral and autonomic repertoires that maintain Tcore during thermal challenges, sensed by thermal receptors, that arise from either the ambient or the internal (e.g., during exercise) environment. A variety of other neural circuits and neurochemical modulators impinge on the fundamental thermoregulatory pathways to produce the alterations in Tcore that occur with circadian and ultradian periodicities1,2 and those that accompany many behavioral states, such as fever during sickness, increased Tcore during psychological stress and at ovulation, and reductions in Tcore during sleep, sepsis, or exposure to metabolic distress (e.g., hypoxia and starvation). Recent research in the central nervous system (CNS) control of Tcore focuses principally on: (a) elaborating the CNS pathways and neurotransmitter systems involved in the fundamental central thermoregulatory network; (b) the modulation of activity within the fundamental central thermoregulatory network by non-thermal factors and behavioral states; and (c) pharmacological manipulation of the CNS thermoregulatory network for a variety of therapeutic goals (e.g., reducing Tcore to produce therapeutic hypothermia in stroke victims).
In its simplest form, the fundamental thermoregulatory network can be modeled as a reflex3,4 in which a central integrative circuit alters the activity of thermoeffector mechanisms in response to an input from the combination of peripheral (i.e., skin) and central (i.e., visceral and brain) thermoreceptors that provides a consolidated assessment of Tcore and, importantly, of imminent threats to Tcore. The primary thermoeffector mechanisms recruited for both cold defense and centrally driven hyperthermias (e.g., fever) include: (a) thermoregulatory behaviors to reduce the loss of heat produced during basal metabolism; (b) cutaneous vasoconstriction (CVC) to conserve heat in the body core and limit heat loss to the environment; and (c) heat production (thermogenesis). The principal sources of metabolic heat production, beyond those contributing to basal metabolic rate (e.g., pumping ions across membranes), are brown adipose tissue (BAT), whose sympathetic neural input fuels mitochondria that shunt proton fluxes into heat production, and shivering behavior in skeletal muscle, dependent on the inefficiency of ATP utilization to generate heat. Effector mechanisms for heat defense include: (a) thermoregulatory behavior to increase heat loss; (b) cutaneous vasodilation, which in humans includes a sympathetic vasodilator outflow5,6, combined with increased cardiac output7 and visceral vasoconstriction8–10 to facilitate core heat loss from the body surface; and (c) evaporative cooling (e.g., sweating).
Apart from important “first responders”, the CNS pathways controlling and mediating thermoregulatory behaviors remain incompletely defined11–15. Along these same lines, most research subjects are furry mammals that are strongly dependent on non-sweating mechanisms for evaporative cooling. Although this has limited the available detail on the CNS pathways regulating human sweating6,16,17, considerable insight has been gained on the CNS regulation of other mechanisms of evaporative cooling, such as panting18–20. Additionally, since most of the basic neuroscience of CNS thermoregulatory pathways has been derived from experiments in rodents, including the exclusively in vitro studies of neurons with intrinsic thermosensitivity, the translation of the conclusions, including the circuit model in Figure 1, to humans must be cautiously undertaken (e.g., 21). Finally, although considerable progress has been achieved in the relatively young field of the neuroscience of thermoregulation, the synthesis (Figure 1) of our understanding of this multifaceted neural network controlling multiple thermoeffectors represents a working model, with the expectation of revisions and added detail.
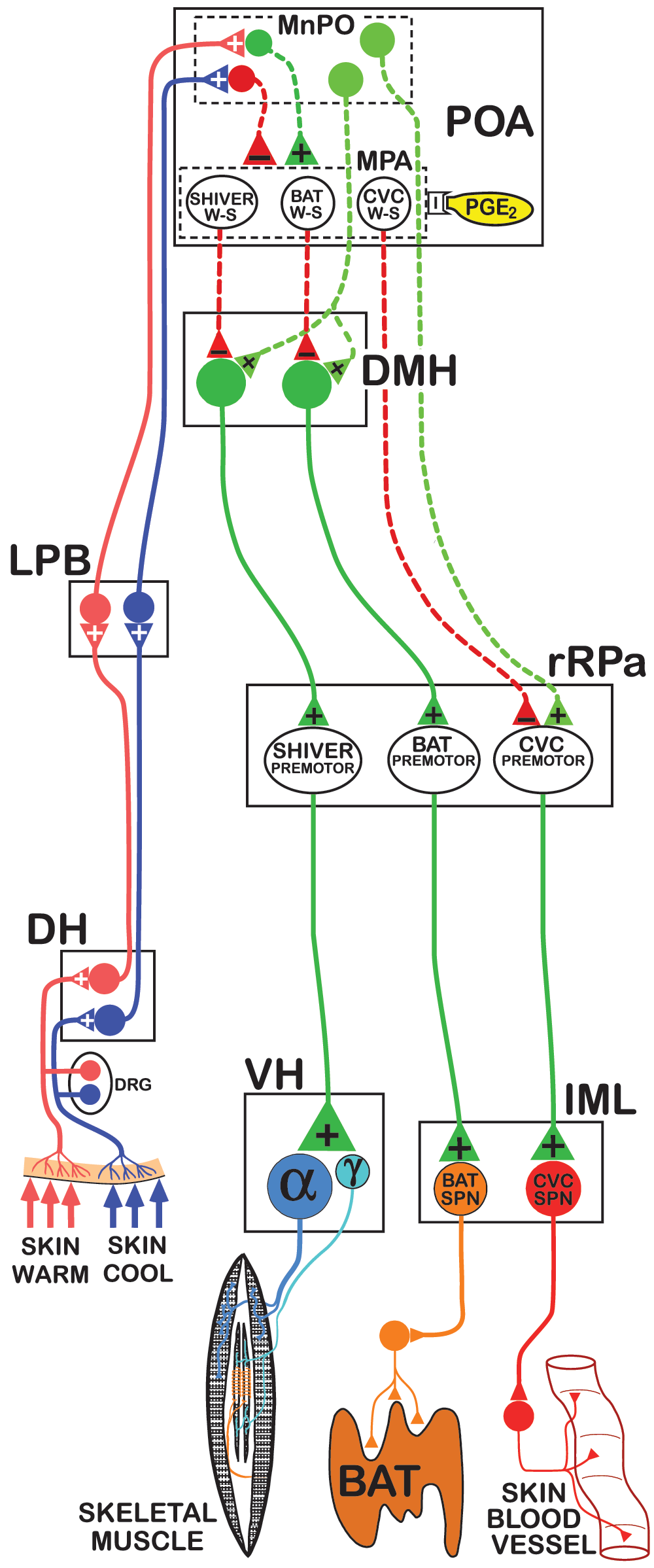
Cool and warm cutaneous thermoreceptors transmit signals to respective primary sensory neurons in the dorsal root ganglia (DRG) which relay this information to second-order thermal sensory neurons in the dorsal horn (DH). Cool sensory DH neurons glutamatergically activate third-order sensory neurons in the external lateral subnucleus of the lateral parabrachial nucleus (LPB), while warm sensory DH neurons project to third-order sensory neurons in the dorsal subnucleus of the LPB. Thermosensory signals driving thermoregulatory responses are transmitted from the LPB to the preoptic area (POA), which contains the microcircuitry through which cutaneous and core thermal signals are integrated to regulate the balance of POA outputs that are excitatory (dashed green) and inhibitory (dashed red) to thermogenesis-promoting neurons in the dorsomedial hypothalamus (DMH) and to CVC sympathetic premotor neurons in the rostral raphe pallidus (rRPa). Within the POA, GABAergic interneurons (red) in the median preoptic (MnPO) subnucleus are postulated to receive a glutamatergic input from skin cooling-activated neurons in LPB and inhibit each of the distinct populations of warm-sensitive (W-S) neurons in the medial preoptic area (MPA) that control CVC, BAT, and shivering. In contrast, glutamatergic interneurons (dark green) in the MnPO are postulated to be excited by glutamatergic inputs from skin warming-activated neurons in LPB and, in turn, excite the populations of W-S neurons in MPA. Prostaglandin E2 (PGE2) binds to EP3 receptors, which are postulated to inhibit the activity of each of the classes of W-S neurons in the POA. Preoptic W-S neurons may provide inhibitory control of CVC by inhibiting CVC sympathetic premotor neurons in the rostral ventromedial medulla, including the rRPa, that project to CVC sympathetic preganglionic neurons (SPNs) in the intermediolateral nucleus (IML). Preoptic W-S neurons may provide inhibitory thermoregulatory control of BAT and shivering thermogenesis by inhibiting BAT sympathoexcitatory neurons and shivering-promoting neurons, respectively, in the DMH, which, when disinhibited during skin and core cooling, provide respective excitatory drives to BAT sympathetic premotor neurons and to skeletal muscle shivering premotor neurons in the rRPa. These, in turn, project, respectively, to BAT SPNs in the IML and to alpha (α) and gamma (γ) motoneurons in the ventral horn (VH) of the spinal cord.
The CNS thermoregulatory control of the sympathetic outflows mediating CVC and BAT thermogenesis and of the somatic motoneurons producing shivering is effected through parallel but distinct, effector-specific, integrative/efferent circuits (Figure 1, and reviewed in 22–25) that share common peripheral thermal sensory inputs. The hypothalamus contains the primary integrative and rostral efferent components of these circuits. Although many details of the preoptic area (POA) microcircuitry for thermoregulation remain to be elucidated, neurons in the POA are postulated to integrate ascending peripheral thermosensory signals with local thermosensitivity to regulate the output of BAT and shivering thermogenesis-promoting neurons in the dorsomedial hypothalamus (DMH)26,27 and of CVC-promoting neurons in the median preoptic nucleus (MnPO)28,29.
The POA regulation of DMH thermogenesis-promoting neurons represents the balance between a GABAergic inhibition30,31 and a glutamatergic excitation32; the latter inputs, potentially arising from neurons in the MnPO that project to the DMH, are synaptically connected to BAT33 and express the leptin receptor34. These glutamatergic inputs to DMH32 could provide the excitation required to drive the BAT sympathoexcitatory neurons and the shivering-promoting neurons in DMH when their POA inhibitory input is reduced during skin cooling or fever35. Although intrinsically warm-sensitive (W-S) (Figure 1), POA neurons36–38, generally located in the medial preoptic area (MPA)39, are postulated to play a key role in central thermoregulation by providing a prominent core temperature-modulated, GABAergic38 regulation of thermogenesis-promoting neurons in DMH (Figure 1), the considerable direct functional evidence required to establish this attractive hypothesis has yet to be obtained. Different thermal sensitivities or neurochemical modulation among populations of temperature-sensitive POA neurons may underlie the differential responsiveness of different effectors to changes in cutaneous versus brain temperatures40 as well as the significant alterations in thermoeffector activation during different sleep phases41. Through their responses to immune signaling molecules, neurons in the POA are also the primary site for the organization and maintenance of the febrile response to inflammation and infection, which includes the stimulation of CVC, and shivering (“chills”) and BAT thermogenesis mediated by the action of prostaglandin E2 (PGE2) on its EP3 receptors42–45. Similarly, the fundamental thermoregulatory network mediates stress-induced hyperthermia46–48. Unraveling the complexity of the thermoregulatory circuitry in the hypothalamus20,28,29,49–52, including the phenotypic characterization of the projection neurons34 and their synaptic interactions that mediate the circadian13 and many behavioral53,54 modulations in Tcore, continues to pose significant research challenges for understanding the “heart” of the CNS thermoregulatory network. The downstream targets of the hypothalamic projection neurons for thermoregulation are the sympathetic and somatic premotor neurons in the rostral ventromedial medulla, centered on the rostral raphe pallidus (rRPa). Midbrain (e.g., periaqueductal gray55–57 and retrorubral field58) and medullary (e.g., ventrolateral medulla and nucleus of the solitary tract (NTS)59,60) pathways to these premotor neurons exert important modulatory influences on the thermoregulatory activation of thermoeffector organs (reviewed in 22,24). These premotor neurons, in turn, excite CVC and BAT sympathetic preganglionic neurons and α-motoneurons and γ-motoneurons61,62 in the spinal cord.
Cold and warm thermoreceptors in the skin and viscera provide the extracranial thermal signals relating to skin temperature and Tcore. These are integrated with the brain temperature information potentially derived from the discharge of W-S, GABAergic preoptic neurons of the central thermoregulatory network to regulate thermoeffector activities. The membranes of thermoreceptor afferent neurons contain transient receptor potential (TRP) cation channels whose temperature-dependent conductances transduce skin temperature into primary thermoreceptor afferent neuronal activity. The TRPM8 channel, activated by menthol and cooling, is the strongest candidate for the cutaneous cold receptor (reviewed in 63). The identity of the peripheral warm receptor remains to be established, but the warm-sensing mechanism of preoptic neurons, though still debated64–66, is unlikely to involve a transient receptor potential (TRP) channel67. Primary thermoreceptor dorsal root ganglion neurons synapse on thermoreceptive-specific, lamina I spinal (or trigeminal) dorsal horn cells68, and these, in turn, collateralize to innervate the thalamus, providing the neural substrate for cutaneous thermosensory perception and localization68,69, and the pontine lateral parabrachial nucleus (LPB)70,71, which is responsible for triggering involuntary (e.g., autonomic and shivering) thermoregulatory responses. Spinal lamina I skin cooling-responsive neurons provide a glutamatergic excitation to neurons in the external lateral subdivision of the lateral parabrachial nucleus (LPBel), which, in turn, project principally to the MnPO of the POA72,73, while a parallel, spinoparabrachial glutamatergic pathway excites POA-projecting neurons in the dorsal subnucleus of the LPB (LPBd)72,74 in response to skin warming74. Thus, activations of POA-projecting LPBd and LPBel neurons, driven respectively by cutaneous, and likely visceral, warm and cold thermoreceptor stimuli, initiate heat defense and cold defense inhibitions and excitations, respectively, in CVC sympathetic outflow and cutaneous blood flow, in BAT sympathetic outflow and BAT thermogenesis, and in shivering EMGs and shivering thermogenesis to maintain a homeostatic Tcore.
Synaptic integration sites throughout the core thermoregulatory network provide the substrate for a wide variety of non-thermal physiological parameters, disease processes, neuromodulators, and drugs to influence the central regulation of Tcore (reviewed in 25). For example, the high metabolic rate of BAT and shivering skeletal muscles during thermogenesis cannot be sustained without a dependable supply of metabolic fuels, particularly oxygen75, lipolytic by-products34, and glucose76. Thus, the CNS networks driving cold-defensive and behavioral BAT activation or shivering are strongly inhibited by signals reflecting a reduction in the short- and long-term availability of these fuel molecules essential for BAT and skeletal muscle metabolism. Similarly, as hypovolemia progresses during dehydration, the ensuing hyperosmolarity reduces the thermoregulatory drive for sweating20,77,78, which serves to prevent cardiovascular collapse79. Some viscerosensory afferents with axons in the vagus nerve and synapsing on second-order neurons in the NTS can also influence BAT activity25,80 and shivering responses and thus are expected to influence the regulation of Tcore. For instance, vagal afferents convey the “metabolic” signals that produce the inhibition of BAT activity induced by upregulation of hepatic glucokinase81 and the BAT activation following either intragastric delivery of the TRP agonist, capsiate82, or the presence of lipids in the duodenum83. Another influence on Tcore includes a prominent hypothermia during motion sickness and nausea84, for which the pathways relating vestibular stimulation to inhibition of CVC and thermogenesis remain to be elaborated.
Providing an additional layer of complexity in the CNS regulation of Tcore is the presence of the thermally responsive TRPV1 channel in the membranes of a variety of classically non-thermal, unmyelinated afferents85–88 that may have access to central thermoregulatory circuits, including via NTS neurons that can inhibit thermogenesis59. The finding that the TRPV1 responsiveness to local brain temperature alters spontaneous glutamate release from the terminals of unmyelinated vagal afferents88,89 provides a potential substrate for Tcore to modulate the activity of second-order sensory neurons in NTS that modulate thermoeffector activation. Importantly, TRPV1 channels are also activated by non-thermal factors, including low (or high) pH, inorganic cations, or endovanilloids, thereby providing a basis for such factors to influence thermoeffector activation and thus Tcore. For instance, TRPV1 channels, potentially on the terminals of afferents in the peritoneum, are stimulated by an endogenous ligand to tonically inhibit BAT thermogenesis, which results in a hyperthermic response to TRPV1 antagonist administration85,90. Although these afferents were not directly tested for their thermal responsiveness, the hyperthermic response to TRPV1 antagonism was not altered by changes in Tcore. The relative influences and the interactions of thermal and non-thermal stimuli on the conductance of the relevant TRPV1 channels could play a role in determining their effect on the level of thermoeffector activation to thermoreceptor stimuli.
Interest in pharmacological modulation of the central thermoregulatory network has focused: (a) on reducing CVC and thermogenesis to lower Tcore; and (b) on augmenting thermogenesis to elevate energy expenditure with the goal of weight loss through consumption of the high-energy lipid stores in white adipose tissue. Novel approaches to reducing Tcore would have immediate benefits in treating intractable fevers that are unresponsive to cyclooxygenase inhibitors. Therapeutic hypothermia can have beneficial effects on survival and on reducing brain and tissue damage in ischemic insults such as cardiac arrest, stroke, and neonatal encephalopathy91–94. Extended space travel (e.g., Mars One) also may require pharmacological induction of a hypothermic, hibernation-like state to reduce energy consumption and psychological stress.
Under basal metabolic and movement conditions, changes in Tcore must arise from changes in the level of activation of thermoeffector tissues. Although modulating neuronal discharge at any site within the central thermoregulatory network would be expected to alter Tcore, centrally generated hyperthermias, such as fever, that arise from altered activity in hypothalamic thermoregulatory neurons could be most effectively reduced by manipulating thermoeffector efferent pathways95,96. Indeed, directly inhibiting the discharge of neurons in the rRPa area, including the functionally significant premotor neurons that control the principal thermoeffectors, produced a fall in brain temperature of approximately 14°C in an ambient environment of 15°C97, and stimulation of α2 adrenergic receptors in the rRPa could completely block or prevent a lipopolysaccharide-evoked fever95. Similarly, central administration of an adenosine A1 receptor (A1AR) agonist reduced rat Tcore by 10°C in an ambient temperature of 15°C60 and mouse Tcore by 5°C in an ambient temperature of 4°C98. Of particular interest, in rats, this hypothermia, which was produced by a blockade of the cold-evoked activation of BAT and shivering thermogenesis, was long-lasting and paralleled by marked reductions in heart and respiratory rate, in EEG, and in behavior60—all reminiscent of those occurring in hibernation and torpor, which require central stimulation of the A1AR99–101. The discovery of a role for TRP channels in thermal sensation and in thermoeffector activation has stimulated research into the pharmacological modulation of TRP channels in the central thermoregulatory network to abrogate the normal cold defense mechanisms and allow Tcore to fall in a cool ambient environment. By reducing the activation of cooling-responsive skin thermoreceptors, a TRPM8 antagonist reduced rat Tcore by approximately 1°C when the rats were in an ambient temperature of 19°C63. Stimulating TRPV1 reduced mouse Tcore by approximately 12°C when mice were exposed to a 10°C ambient temperature102, and this effect was potentiated by the addition of a TRPM8 antagonist103. The location of the relevant, hypothermic TRPV1 channels remains unknown.
Not only is BAT a thermogenic thermoeffector, including in adult humans104–107, but through its consumption of lipid and glucose energy stores and oxygen, thermogenic metabolism in BAT is a neurally regulated contributor to energy homeostasis. Thus, particularly in the face of an elevated consumption of energy-rich food (e.g., a high-fat diet), a chronic reduction in cooling-evoked BAT thermogenesis would contribute to the augmented adipose energy stores that characterize obesity. Indeed, mice without BAT exhibit a propensity for obesity and diabetes108,109; conversely, overexpression of uncoupling protein-1 (UCP-1), principally responsible for thermogenesis in BAT, mitigates obesity induced by a high-fat diet110. Several anti-obesity therapies currently being explored are based on increased activation of BAT thermogenesis, through either activation of the central thermoregulatory network to increase the sympathetic outflow to BAT111 or an alteration in the cellular biochemical pathways in brown adipocytes or a hyperplasia of BAT to augment thermogenesis. The consistent findings that obese humans have significantly reduced cooling-activated BAT104–106,112, and that the basal (i.e., principally cooling-evoked) sympathetic activation of BAT is reduced in rats fed a high-fat diet113,114, and that a vagal afferent input to the NTS mediates the reduced cooling-evoked BAT activity in rats fed a high-fat diet115 not only support a role for reduced BAT activity in the excess adipose accumulation of obesity but also highlight the significance of non-thermal inputs to the central thermoregulatory network25,81,83 in influencing even the most basic thermoregulatory responses.
Considerable progress has been achieved in revealing the functional organization of the dedicated thermoregulatory network within the CNS that provides the fundamental neural control of the thermoregulatory effectors: thermoregulatory behavior, CVC, and BAT and shivering thermogenesis, although many of the details of the neurophysiology and neuroanatomy of the central thermoregulatory network remain active areas of investigation. The changes in Tcore that accompany a wide range of behaviors and in response to many hormones and drugs arise through altered non-thermal inputs to, or neurochemical modulation of, the neural activity within the fundamental thermoregulatory network. The latter, as well as thermoreceptor-based strategies, are being researched as therapeutic approaches in which the central thermoregulatory networks are recruited to alter Tcore and metabolism.
This work was supported by National Institutes of Health grants R01NS040987 and R01NS091066 to Shaun F. Morrison.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 12 May 16 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)