Keywords
prostate specific antigen, elite female athletes, hyperandrogenism, fifth-generation PSA assays, serum PSA in women, Olympic teams,
prostate specific antigen, elite female athletes, hyperandrogenism, fifth-generation PSA assays, serum PSA in women, Olympic teams,
Prostate specific antigen (PSA) is a well-known and clinically useful biomarker of prostate adenocarcinoma1. PSA circulates in blood of males as a complex with alpha 1 antichymotrypsin (cPSA) (approx. 80% of total) or as free, non-complexed PSA (fPSA) (approx. 20% of total)2,3. It has now been well-documented that PSA is also produced by many female tissues, including breast, periurethral, salivary and thyroid tissues, and by many tumors4,5. The PSA gene is up-regulated by androgens and progestins in breast and other female tissues, as well as in model systems such as breast carcinoma cell lines6–12. Serum PSA in women fluctuates during the menstrual cycle, and these changes are attributed to up-regulation by progesterone during the luteal phase13–15.
PSA also circulates in female serum as complexed and free PSA16, but its concentrations are exceedingly low (around 1pg/mL), precluding accurate determination with third generation PSA assays17,18. In some circumstances, such as in women with hyperandrogenic syndromes, including polycystic ovarian syndrome (PCOS) and hirsutism, it has been shown that total PSA in female serum is elevated and this finding may be used as an aid to disease diagnosis19–25.
Recently, fifth generation PSA assays have been developed by many groups, allowing accurate PSA determinations in the low fg/mL range26–31. These assays can quantify both complexed and free PSA in serum and urine of females and allow examination of the possible role of female PSA in healthy and disease states. We recently confirmed the diagnostic value of cPSA and fPSA in women with PCOS by using one of these assays32.
It has previously been speculated that female elite athletes may have higher circulating androgen levels than sedentary age-matched females, and that hyperandrogenic syndromes such as PCOS, congenital adrenal hyperplasia and 46XY disorders of sex development are more common in elite athletes33–37.
In this paper we speculated that serum cPSA and fPSA, due to their in-vivo up-regulation by androgens and progestins, as seen with female-to-male transsexuals treated with testosterone38,39, may represent an integrated index of total androgenic/progestational activity in female tissues. We thus examine here, the levels of serum complexed and free PSA in 106 female elite athletes and 114 age-matched sedentary controls, along with levels of estrogens, androgens and progesterone. The observed differences in serum cPSA and fPSA between athletes and controls were examined in the context of oral contraceptive use and ovulatory cycles.
This project was approved by the Regional Ethics Committee of the Karolinska Institutet, Stockholm, Sweden (EPN 2011-1426-3). Serum samples from 106 Swedish Olympic female athletes representative of the Swedish participation in the summer and winter Olympic games, were recruited in connection with pre-Olympic training camps. Participants were at least 18 years of age. Samples were also collected from 114 age-matched, healthy non-athletic female controls (maximum of 2 hours endurance and/or strength training per week and no prior participation in elite level competition). Recruitment started in November, 2011 and was completed by April, 2015.
The subjects were investigated at the Women’s Health Research Unit, Karolinska University Hospital or in connection with training camps. Data on ethnicity, past and present health problems, injuries, medications, gynecological history (bleeding pattern, date of last menstruation, pregnancies, hormonal contraceptive use) and symptoms of hyperandrogenism (hirsutism, acne), were collected by a general health questionnaire. Furthermore, data on sport discipline, training hours per week, achieved sport performance and goals were collected from the Olympic athletes. A fasting blood sample was collected by a standard venipuncture between 7–9AM. Body composition (body fat, muscle mass, BMD) was investigated by dual X-ray absorptiometry (DXA), at the Department of Radiology, Karolinska University Hospital, Solna, Stockholm.
Serum from both athletes and controls was analyzed by tandem mass spectrometry for the following steroid hormones and metabolites: estrone (E1), estradiol (E2), testosterone (Testo), dehydroepiandrosterone (DHEA), androstenedione (Delta 4), androst-5-ene-3β, 17β-diol (Delta 5), dihydrotestosterone (DHT), and progesterone (PROG).
Serum samples were kept frozen at -80°C until thawed for analysis. Details of the tandem mass spectrometry analysis have been described elsewhere40. A progesterone concentration ≥ 5.3 ng/mL in the luteal phase of the menstrual cycle was taken as an indication of successful ovulation.
Among the athletes, 65 were not using contraceptives and 41 were using hormonal contraceptives (39%). Among the control group, 69 were not using contraceptives and 45 were using contraceptives (39%). The type of oral contraceptive used was quite variable and included the following combinations: Ethinylestradiol + Levonorgestrel, Ethinylestradiol + Etonogestrel, Ethinylestradiol + Drospirenon, Ethinylestradiol + Dienogest, Ethinylestradiol + Norgestimate, Ethinylestradiol + Cyproterone, Ethinylestradiol + Nomegestrol, Ethinylestradiol + Noretisterone, or the following single progestins: Desogestrel, Etonogestrel, Levonorgestrel. Due to the small number of participants, we did not analyze the data according to type of contraceptive used.
One vial of 200µL serum per sample was provided blinded to Meso Scale Diagnostics (MSD) for cPSA and fPSA measurement using MSD’s MULTI-ARRAY® electrochemiluminescence technology in the S-PLEXTM format, which allows quantitation of previously unmeasurable levels of biomarkers with fg/mL sensitivity27,41. The samples were thawed and centrifuged at 10,000g for 10 minutes at 4˚C before being aliquoted into low retention 96-well round bottom plates for subsequent testing. Plates were immediately frozen on dry ice and stored at -80˚C until testing. cPSA and fPSA assays were calibrated to the WHO International Standard for prostate-specific antigen, with 90% bound to alpha1-antichymotrypsin and 10% in the free form (National Institute for Biological Standards and Control, [NIBSC], code 96/670, Hertfordshire, England) and the WHO International Standard for prostate-specific antigen free (NIBSC, code 96/668, Hertfordshire, England), respectively. Assay characteristics were determined prior to sample testing.
For each assay, 8-point calibration curves were included on each plate, and the data were fitted with a weighted 4-parameter logistic curve fit. Limit of detection (LOD) is a calculated concentration corresponding to the average signal 2.5 standard deviations above the background (zero calibrator). Lower limit of quantitation (LLOQ) and upper limit of quantitation (ULOQ) were established for the plate lot by measuring multiple levels of calibrator near the expected LLOQ and ULOQ. LLOQ and ULOQ are, respectively, the lowest and highest concentration of calibrator tested which has a %CV of 20% or less, with recovered concentration within 70–130%. The LOD was 5.7 and 140 fg/mL for cPSA and fPSA assays, respectively. The LLOQ was 17 and 480 fg/mL for cPSA and fPSA assays, respectively. The ULOQ was 72,000 and 240,0000 fg/mL for cPSA and fPSA assays, respectively. Precision was determined from testing of three internal quality control samples that span the detectable range and is expressed as the % CV from 16 specimen assay runs, with 2 operators over 3 testing days. % CVs were between 11–13% for cPSA and 6–23% for fPSA.
Serum, EDTA plasma, and heparin plasma samples (7–8 samples total) were spiked with calibrator at two or three concentrations. The non-complexing form of PSA (Scripps Laboratories, San Diego, CA; #90024) that does not bind to a1- antichymotrypsin (ACT) was used in spike recovery experiments for the fPSA assay. Average spike recoveries for the fPSA and cPSA assays were 88% and 90%, respectively. Serum, EDTA plasma, and heparin plasma samples (7–8 samples total) were diluted 2, 4 and 8-fold. Average dilution linearities for the fPSA and cPSA assays were 114% and 109%, respectively.
The samples and calibrator dilutions were assayed in duplicate and all samples were measured for cPSA and fPSA. Measurement of cPSA was performed with a 2-fold dilution of the samples; fPSA measurement was performed on neat samples. Concentrations of biomarkers in each sample were calculated from the calibrator curves taking into account sample dilutions. The mean of two measurements was derived for each analyte in each sample and reported in fg/mL.
We first categorized the athletes and control subjects by several clinical and demographic variables and hormonal measurements. When comparing variables among athletes and controls, the Wilcoxon rank sum test was used to determine if there were significant differences. Correlation of parameters between athletes and controls were examined using the Spearman correlation coefficient.
Lower limits of detection (LOD) for cPSA and fPSA assays are 6 fg/mL and 140 fg/mL, respectively. Since measurements that fall below these values are unreliable, we set marker measurements that fall below the LOD to LOD/2. Among all samples, 102/206 serum fPSA values but none of the serum cPSA values were below the LOD of the method used. Similarly, 110 out of 220 progesterone values were below the detection limit of the method (0.10 ng/mL) and these values were adjusted to 0.05 ng/mL. Rank-based non-parametric methods were employed so that the results would be robust to these transformations.
The included excel file contains all anthopometric and biochemical data used to perform statistical analyses. Significant differences between athletes and controls, irrespective of oral contraceptive use, were noted for the following variables: total BMD, spine BMD, lean mass (all parameters were elevated in athletes; p<0.001) and fat percentage (decreased in athletes; p<0.001).
Initial examination of our data revealed the significant effect of oral contraceptives on hormonal and PSA measurements. For this reason, we stratified both athlete and control groups by hormonal measurements and PSA, according to oral contraceptive use (data shown in Table 1 and Table 2). E1 and E2 levels were significantly reduced in athletes (p=0.003 and 0.004, respectively). Other measurements were not significantly different between athletes and controls that were not taking oral contraceptives, but there was a trend for athletes to have higher DHEA (p=0.095) and Delta 5 (p=0.084). In Table 2 it is shown that both cPSA and fPSA were significantly lower in athletes in comparison to control subjects, irrespective of hormonal contraceptive use. Median cPSA was 776 fg/mL in athletes and 1249 fg/mL in controls (p=0.003). Median fPSA was 70 fg/mL in athletes and 169.5 fg/mL in controls (p=0.013).
| Athletes (n = 106) | Controls (n = 114) | |||||||
|---|---|---|---|---|---|---|---|---|
| No Hormonal Contraceptives | Hormonal Contraceptives | No Hormonal Contraceptives | Hormonal Contraceptives | |||||
| n2 | n | n | n | |||||
| Age (years) | 65 | 26 (22, 30) | 41 | 24 (22, 27) | 69 | 25 (23, 30) | 45 | 24 (22, 26) |
| Weight (kg) | 64 | 65 (60, 70.2) | 41 | 62 (60, 68.7) | 69 | 60 (56, 68) | 45 | 62 (55.5, 68) |
| Total BMD (g/cm3) | 41 | 1.25 (1.19, 1.31) | 24 | 1.23 (1.16, 1.31) | 59 | 1.15 (1.08, 1.2) | 39 | 1.13 (1.1, 1.18) |
| Spine BMD (g/cm) | 41 | 1.12 (1.01, 1.19) | 24 | 1.1 (1.03, 1.19) | 59 | 1.02 (0.94, 1.07) | 39 | 0.99 (0.95, 1.03) |
| Fat % | 41 | 17.2 (13.3, 22.6) | 24 | 19.7 (15.6, 21.6) | 59 | 31 (27.2, 35.5) | 39 | 32.1 (26.9, 36.6) |
| thorax/total (fat) | 40 | 0.45 (0.42, 0.47) | 24 | 0.43 (0.42, 0.46) | 59 | 0.45 (0.41, 0.49) | 39 | 0.44 (0.41, 0.48) |
| bone/total (fat) | 40 | 0.43 (0.41, 0.45) | 24 | 0.45 (0.41, 0.46) | 59 | 0.42 (0.4, 0.46) | 39 | 0.43 (0.41, 0.46) |
| lean mass total (kg) | 41 | 50.2 (46.4, 53.2) | 24 | 48.9 (47.1, 52.6) | 59 | 39.5 (37.7, 42.8) | 39 | 40.6 (37.3, 43.6) |
| lean mass legs (kg) | 41 | 17.7 (15.5, 18.6) | 24 | 17.4 (16.3, 18.8) | 59 | 13.6 (12.5, 14.3) | 39 | 13.8 (12.4, 15.3) |
| total mass (kg) | 41 | 64.2 (59.5, 67.8) | 24 | 63.6 (60.5, 70.9) | 59 | 61.1 (55.7, 66.6) | 39 | 63.9 (55.9, 69.5) |
| E1 (pg/mL)1 | 65 | 43.4 (34, 65.5) | 41 | 23 (16.3, 33.8) | 69 | 60.1 (40.5, 89.4) | 45 | 23.9 (18.9, 34.1) |
| E2 (pg/mL) | 65 | 67 (32.3, 106.5) | 41 | 13.1 (4.2, 30) | 69 | 109.9 (45.7, 161.9) | 45 | 10.1 (3.6, 33.4) |
| DHEA (ng/mL) | 65 | 7.7 (5.4, 10.9) | 41 | 6.5 (4.1, 9.8) | 69 | 6.1 (4.7, 8.9) | 45 | 5 (4, 5.9) |
| Testo (pg/mL) | 65 | 280 (218, 378) | 41 | 235 (166, 345) | 69 | 282 (242, 348) | 45 | 265 (185, 314) |
| Delta4 (pg/mL) | 65 | 1411 (1069, 1577) | 41 | 987 (755, 1236) | 69 | 1379 (1063, 1765) | 45 | 891 (711, 1113) |
| Delta5 (pg/mL) | 65 | 717 (529, 930) | 40 | 672 (458, 826) | 69 | 619 (479, 801) | 45 | 572 (435, 731) |
| DHT (pg/mL) | 65 | 107.6 (79.6, 138.3) | 41 | 98.6 (74.6, 121.4) | 69 | 103.4 (87.8, 168.7) | 45 | 97.8 (77.5, 140.4) |
| PROG (ng/mL) | 65 | 0.16 (0.11, 1.19) | 41 | 0.05 (0.05, 0.11) | 69 | 0.13 (0.05, 7.18) | 45 | 0.05 (0.05, 0.05) |
| PROG > 5.3 ng/mL | 65 | 10 (15%) | 41 | 1 (2%) | 69 | 19 (28%) | 45 | 0 (0%) |
| cPSA (serum), fg/mL | 63 | 776 (353.5, 2165) | 40 | 1812 (579, 3236) | 68 | 1249 (509.8, 3985.5) | 45 | 2002 (690, 2369) |
| fPSA (serum), fg/mL | 63 | 70 (70, 216.5) | 40 | 216.5 (70, 525.2) | 68 | 169.5 (70, 394.8) | 45 | 220 (70, 379) |
| Athletes (n = 65) | Controls (n =69) | p-value1 | |||
|---|---|---|---|---|---|
| E1 (pg/mL) | 43.4 | (34, 65.5) | 60.1 | (40.5, 89.4) | 0.003 |
| E2 (pg/mL) | 67 | (32.3, 106.5) | 109.9 | (45.7, 161.9) | 0.004 |
| DHEA (ng/mL) | 7.7 | (5.4, 10.9) | 6.1 | (4.7, 8.9) | 0.095 |
| Testo (pg/mL) | 280 | (218, 378) | 282 | (242, 348) | 0.831 |
| Delta 4 (pg/mL) | 1411 | (1069, 1577) | 1379 | (1063, 1765) | 0.947 |
| Delta5 (pg/mL) | 717 | (529, 930) | 619 | (479, 801) | 0.084 |
| DHT (pg/mL) | 107.6 | (79.6, 138.3) | 103.4 | (87.8, 168.7) | 0.257 |
| PROG (ng/mL) | 0.16 | (0.11, 1.19) | 0.13 | (0.05, 7.18) | 0.7 |
| PROG ≥ 5.3 ng/mL | 10/55 (15%) | 19/50 (28%) | 0.134 | ||
| cPSA (serum) fg/mL | 776 | (353.5, 2165) | 1249 | (509.8, 3985.5) | 0.033 |
| fPSA (serum) fg/mL | 70 | (70, 216.5) | 169.5 | (70, 394.8) | 0.013 |
It is shown in Table 3 that in athletes, the use of oral contraceptives significantly increased both cPSA (from 776 to 1812 fg/mL; (p=0.046) and fPSA from 70 to 216 fg/mL (p=0.009). In control subjects, we noticed similar trends (from 1249 fg/mL to 2002 fg/mL for cPSA and from 169.5 fg/mL to 220 fg/mL for fPSA) but the differences were not significant. The elevation of serum PSA with oral contraceptive use in women has been reported before9.
Numbers represent medians (25th, 75th percentiles).
| No Hormonal Contraceptives | Hormonal Contraceptives | p-value1 | |||
|---|---|---|---|---|---|
| Athletes | |||||
| cPSA (fg/mL) | 776 | (353.5, 2165)2 | 1812 | (579, 3236) | 0.04628 |
| fPSA (fg/mL) | 70 | (70, 216.5) | 216.5 | (70, 525.2) | 0.00917 |
| Controls | |||||
| cPSA (fg/mL) | 1249 | (509.8, 3985.5) | 2002 | (690, 2369) | 0.62432 |
| fPSA (fg/mL) | 169.5 | (70, 394.8) | 220 | (70, 379) | 0.53344 |
The distribution of all clinical and demographic variables between athletes and controls, stratified by oral contraceptive use, is shown in Supplementary Figure 1. The distribution of all hormonal and PSA measurements in controls and athletes, stratified by oral contraceptive use, is shown in Supplementary Figure 2 and Supplementary Figure 3.
We then examined the correlation between cPSA and fPSA in serum of both athletes and controls. The Spearman correlation coefficients (rs) were highly significant for both groups of subjects (Figure 1). For athletes, rs=0.75 (p < 0.001) and for controls, rs=0.64 (p < 0.001). We also examined the correlation between the two PSA variables and all other clinical, demographic and hormonal variables in subjects not taking oral contraceptives (Supplementary Table 1). The only statistically significant correlations (p < 0.01; all positive) were between cPSA and fPSA and age, and cPSA with Testo, Delta4 and Delta5 in the athletes group, and between cPSA and fPSA with DHEA, Testo, Delta4 and Delta5 in the control group. The correlations between all of the available parameters in the control and athlete groups not taking oral contraceptives are further depicted in Figure 2.
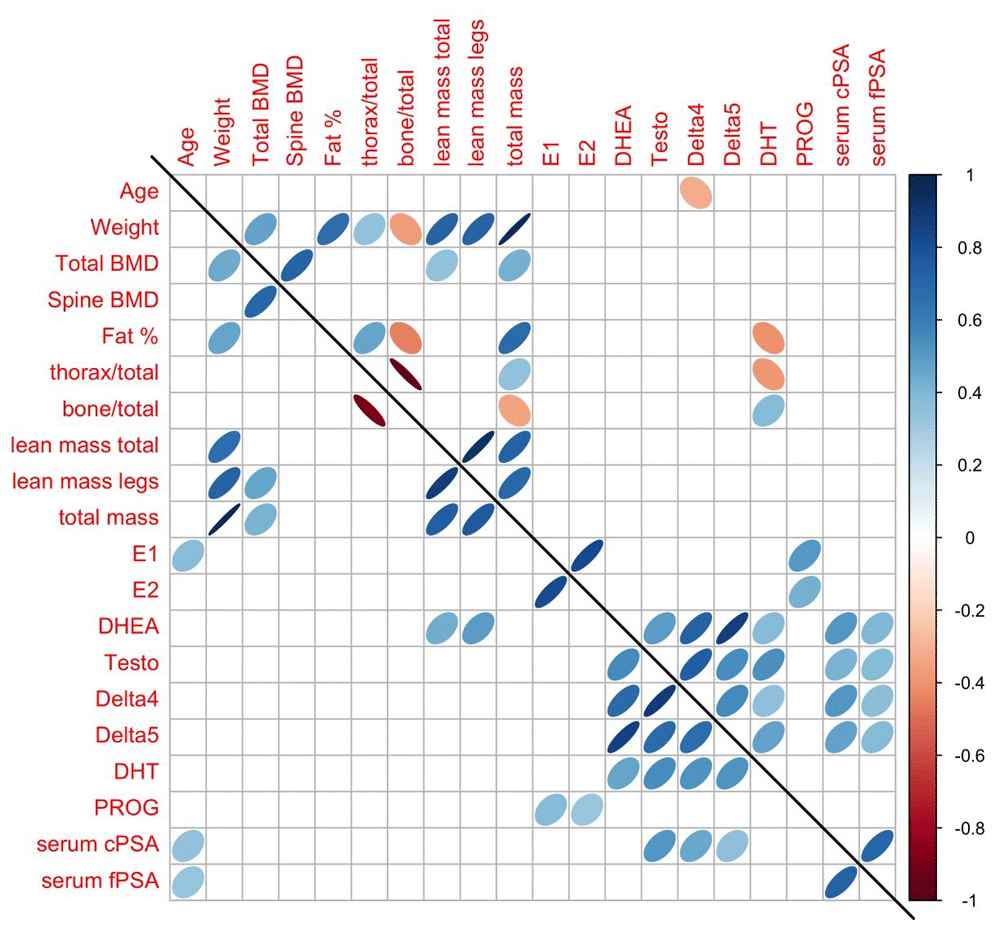
The pairs for which ovals are displayed indicate a Spearman correlation that is significantly different from zero, with p-value < 0.01. See also Table 1 for parameter description.
We also examined whether cPSA and fPSA correlated with progesterone. We found no correlation, in either the control or athlete groups, as stratified by hormonal contraceptive use (Supplementary Figure 4). We further dichotomously classified progesterone as being ≥ 5.3 ng/mL (indicative of luteal phase) or < 5.3 ng/mL in athletes and controls not using oral contraceptives, and examined any association with cPSA or fPSA. We found no association (Supplementary Figure 5).
PSA is used widely for diagnosis and monitoring of prostatic adenocarcinoma in males1. Quantification of PSA in female serum is problematic, due to its very low concentrations4,5. Newly developed fifth generation PSA assays demonstrate enough sensitivity for quantifying both complexed PSA and free PSA in serum of normal women27. We have recently shown that median cPSA concentration in normal women is around 900 fg/mL, while fPSA is about ten times lower (70 fg/mL). In women with PCOS, serum PSA concentration is elevated by about 3-fold32.
It is well-known that the PSA gene is up-regulated by androgens7–12. Previous studies have shown that women who are taking androgenic steroids to change sex (e.g. young female-to-male transsexuals) demonstrate high elevations of serum PSA and urine PSA37,38. We have also shown previously that women who are taking oral contraceptives upregulate their PSA at both the tissue and the serum level9. Based on this data, we hypothesized that serum PSA concentration may represent a novel integrated index of androgenic stimulation in normal women.
It has been suggested that elite athletes more frequently suffer from various hyperandrogenic syndromes such as PCOS, congenital adrenal hyperplasia and 46XY disorders of sex development33–37. In this paper, we examine the possible differences in serum PSA between Olympic elite athletes and sedentary control women. For this, we measured both cPSA and fPSA, as well as a panel of androgens, estrogens, and progesterone in serum.
We have shown that in athletes, E1 and E2 concentrations are significantly reduced, whereas levels of DHEA and Delta 5 tend to be slightly elevated. Moreover, we identified for the first time a significant decrease in serum cPSA and fPSA in elite athletes, in comparison to the control group. We have also confirmed the previous finding9 that oral contraceptive use increases both cPSA and fPSA in serum of athletes (but the differences were not significant in the control group, although the same trend was observed).
Recently, we speculated that serum and urine PSA in female athletes could be used to test for doping with androgenic steroids42. In this work, we did not examine this possibility due to lack of samples from provenly doping athletes. However, now that serum PSA in women can be reliably quantified, this parameter could be considered for inclusion into the athlete’s biological passport, as suggested by Sottas et al43.
Our finding that serum E1 and E2 are lower in athletes or exercising individuals in comparison to controls is supported by previous literature43–45. An explanation for this phenomenon may be related to menstrual cycle disturbances induced by strenuous exercise and stress during the competition. Such findings have prompted some to speculate that the lower incidence of breast cancer in individuals who exercise may be due to reduced estrogen and androgen levels43–45. Previously, we also established a connection between tumoral or nipple aspirate PSA and breast cancer prognosis46–49. Clearly, the interconnections between exercise, androgen, estrogen and serum PSA levels and breast cancer need to be better defined.
It has been reported before that serum PSA fluctuates with the menstrual cycle, likely due to up-regulation by progesterone in the luteal phase13–15. We examined the correlation and the association of cPSA and fPSA with serum progesterone, as a continuous and dichotomous variable, respectively. Our results have shown that there was no correlation or association between either cPSA or fPSA and progesterone or with the frequency of ovulatory cycles, (ovulation being defined as progesterone ≥ 5.4 ng/mL in the luteal phase).
While we found that many anthropometric measurements are different between athletes and controls (Table 1), among steroid measurements, only E1, E2, and to a lesser extent DHEA and Delta5 concentrations were different between the two groups. However, we identified a significant difference between both cPSA and fPSA between elite athletes and controls. The differences in cPSA and fPSA levels between these two groups do not seem to be associated with hyperandrogenism or the menstrual cycle. It will be interesting to examine, in the future, what other parameters are responsible for the differential concentrations of cPSA and fPSA in serum of these women. Since PSA in women originates mostly from the breast, differences in breast size, which was one parameter we did not study, could be one possible cause for the different levels of these proteins.
Dataset 1: Anthropometric and biochemical data for athletes and controls. The data file contains all available anthropometric and biochemical data for all athletes and controls included in this study, and includes free PSA and complexed PSA. It was used to do statistics and to derive the results of this paper. DOI, 10.5256/f1000research.11821.d16803950
This study was approved by the Regional Ethics Committee of the Karolinska Institutet, Stockholm, Sweden (EPN 2011-1426-3). Written informed consent was obtained from all participants.
Authors SW, AM, MS, EG and GN are employees of Meso Scale Diagnostics. The other authors have no conflicts of interest to declare.
This work was funded by the Partnership for Clean Competition (grant to EPD).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Figure 1: Distribution of age, weight and body composition variables between athletes and controls, stratified by oral contraceptive use.
Click here to access the data.
Supplementary Figure 2: Distributions of steroid hormone variables among athletes and controls, stratified by oral contraceptive use.
Click here to access the data.
Supplementary Figure 3: Distributions of serum PSA measurements among athletes and controls, stratified by oral contraceptive use.
Click here to access the data.
Supplementary Figure 4: Correlation between PSA values and progesterone in athletes and controls, stratified by hormonal contraceptive use. No correlation was found.
Click here to access the data.
Supplementary Figure 5: PSA values stratified by progesterone < 5.3 vs. ≥ 5.4 ng/mL in athletes and controls, for women who do not take hormonal contraceptives. P-values are calculated using Wilcoxon tests.
Click here to access the data.
Supplementary Table 1: Spearman correlation between PSA and other variables among athletes and controls for women not taking hormonal contraceptives. The coloured cells indicate a Spearman correlation that is significantly different from zero with p-value < 0.01.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 17 Jul 17 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)