Keywords
Aflatoxin B1, apoptosis, Schiff bases amino acid derivatives, immune system, antioxidant system
Aflatoxin B1, apoptosis, Schiff bases amino acid derivatives, immune system, antioxidant system
Aflatoxin B1 (AFB1) is a mycotoxin produced by Aspergillus flavus and related fungi that grow in staple foods, including cereals and nuts, such as corn, rice and peanuts1, especially in areas with appropriate conditions of moisture and heat where these fungi are ubiquitous. AFB1 causes a serious threat for human and animal health and, in extreme cases, lead to death2. The toxicity of AFB1 has been shown to be associated with a wide range of pathological events such as enhanced apoptosis, oxidative events and carcinogenesis3–5. Moreover, AFB1 is the one of mycotoxins that were adopted for the use in bioterrorism6–7.
AFB1 is metabolized into aflatoxin-8,9-epoxide, reactive oxygen species (ROS)8–9, which can react with proteins and DNA to form adducts and cause mutations in the p53 and other genes essential for cell malignant transformation3,10. AFB1 has also been shown to be immunotoxic to animals and is suspected to be immunosuppressive in humans11–14.
Currently there is no treatment for mycotoxin exposure, except of supporting therapy, such as diet and hydration15. Development of the efficient measures neutralization of toxic effects of mycotoxins and prevention of associated pathological changes is an issue of great importance. The key aspect here is that the potential therapeutics should possess multi-mechanistic actions and simultaneously target a range of pathological processes caused by AFB1.
Our previous studies have demonstrated that Schiff base amino acid derivatives picolinyl-L-phenylalaninate (PLP), picolinyl-L-tryptophanate (PLT) and nicotinyl-L-tryptophanate (NLT) are capable of scavenging free-radicals, elevating the capacities of antioxidant and the immune system in radiation injury, and possessing anticytotoxic, antigenotoxic and antimutagenic properties16,17. Based on these results, we suggest that the above-mentioned multifunctional compounds may have protective effects against mycotoxins. This suggestion is also supported by other results indicating that several Schiff base derivatives are capable of decreasing the concentrations of aflatoxin M1 in artificially contaminated raw milk18, and have good neutralization activity against Aspergillus niger19. In addition, L-tryptophan was shown to alleviate aflatoxin-induced chicken growth retardation and immunosuppression20, while phenylalanine may prevent ochratoxin A-induced suppression of the immune response21 and inhibition of protein synthesis in spleen, kidney and liver22.
This study is aimed to evaluate the ability of novel Schiff base cyclic amino acid derivatives to protect against oxidative stress and immunosuppression in an animal model of AFB1 mycotoxicosis.
The synthesis of PLT was performed as described previously17 by condensation of picolinaldehyde (2-pyridinecarboxaldehyde) and potassium salt of L-tryptophan in alcohol solution (ethanol, methanol) in a molar ratio 1:1 at the temperature range 5–25°C. A similar procedure was used for NLT synthesis, which is a condensation product of nicotinaldehyde (3-pyridinecarboxaldehyde) and L-tryptophan potassium salt, and PLP, which is a condensation product of picolinaldehyde (2-pyridinecarboxaldehyde) and L-phenylalanine potassium salt (Figure 1).
Mongrel white pubescent male rats (180–200 g; Animal Facility of the Institute of Molceular Biology, National Academy of Sciences, Armenia) were used in all experiments. Twelve randomly selected animals were used in each of the following groups: 1) Controls – no treatment; 2) AFB1 mycotoxicosis – rats orally treated with AFB1 mycotoxin during 21 days at 25μg/kg per day dose level and left for 10 additional days without any treatment; 3) AFB1 mycotoxicosis + Schiff bases – rats orally treated with AFB1 mycotoxin during 21 days at 25μg/kg dose level and 10-day oral treatment with PLP, PLT or NLT at 10 mg/kg per day dose level; 4) Schiff-base only – rats received 10-day oral treatment with 10mg/kg PLP, PLT or NLT. Oral treatment with AFB1 and Schiff bases was delivered with water. At the end of the treatment period, animals were euthanized by decapitation.
During the treatment period animals were allowed free access to water and food and were kept (maximum 5 animals per cage) in pathogen free conditions at regular 12 hour day-night cycles. Animal care, handling and use in research were performed according to the international regulations adopted by the Ministry of Health of the Republic of Armenia. The described experimental protocols of animal studies were considered and approved by the Ethical Committee acting at Institute of Molecular Biology. All efforts were made to ameliorate any suffering of the animals; animal decapitation was performed in dedicated room located distantly from animal care facility by trained personnel using sharpened guillotines regularly adjusted to ensure proper performance.
Fresh trunk blood samples were collected in EDTA containing tubes during decapitation. Fresh blood aliquots were immediately used for flow cytometry (see below). Plasma was separated with centrifugation (10 minutes at 3000g at 4°C) and stored at -30°C until further analyses.
Malonic dialdehyde (MDA) content, as a marker of terminal phase of lipid peroxidation, was measured in blood plasma (0.1 mL)23 and liver homogenate. Homogenate was obtained by excising 100 mg of liver and pulverizing in solution containing 0.3 mL 40mM Tris-HCl buffer (pH = 7.4), 0.3 mL 12*10-6 M Mohr salt and 0.3 mL of 0.8 mM of ascorbic acid24. The activity of lipid peroxidation in blood plasma and liver is based on the amount of MDA formation, which during interaction with 0.8 mL 0.12M thiobarbituric acid, gives a coloring reaction, determined at a wavelength 535 nm using UV-752 UV-VIS Spectrophotometer (Shanghai Phenix Optical Scientific Instrument Co. Ltd, China). Optical density was converted to concentration units using Excel 2007. The MDA concentration was calculated using an extinction coefficient and expressed as µMol MDA/g liver tissue or µMol MDA/mL plasma.
The integral antioxidant activity (AOA) represents the sum of the antioxidant capacity of hydrophilic and lipophilic antioxidants of low-molecular non-enzymatic water-soluble antioxidants (ascorbic acid, glutathione and uric acid) of blood serum. AOA was analyzed by photochemiluminescence detection using a Photochem analyzer and ACW Kit (Analytik Jena AG, Jena, Germany), as per the manufacturer’s instructions. In this assay, generation of free radicals is partially eliminated by the reaction with the antioxidants present in the serum samples, and the remaining radicals are quantified by luminescence generation. An ascorbate calibration curve was used to evaluate AOA levels. The results were expressed as conventional units equal to mMol ascorbate with equivalent activity.
Plasma levels of circulating immune complexes (CIC) (Rat Circulating Immune Complexes ELISA kit; BlueGene Biotech, Shanghai, China), terminal complement complex (C5b-C9) (Rat Terminal complement complex (C5b-9) ELISA kit; BlueGene Biotech), superoxide dismutase (SOD) (Rat Superoxide Dismutase Copper (SOD) ELISA kit; BlueGene Biotech), and catalase (CAT) (Rat Catalase ELISA kit; BlueGene Biotech) were measured using commercially available ELISA kits, according to manufacturer’s instructions using StatFax-2100 plate reader (Awareness Technology Inc, USA). The detection limit for CICs, C5b9, SOD, CAT was 0.1ng/mL, 0.1pg/mL, 0.1µg/mL, 0.1ng/mL, respectively.
100 μL of whole blood from each studied rat was used to quantify apoptotic rate and percentage of non-viable cells. Erythrocytes were discarded by lysis (ammonium chloride lysis buffer); white blood cells were washed in Annexin-binding buffer and stained with 5 μL of Annexin V–FITC conjugate for 20 minutes, followed by staining with 1 μg/mL of propidium iodide (PI). Apoptotic rate and cell viability were analyzed on a Partec CyFlow Space (Partec, Germany). 10 000 events were collected from each sample. The neutrophil and monocyte populations in peripheral blood were distinguished by forward scatter and side scatter. Gating and determination of early and late apoptotic rates were done by FlowJo vX0.7 software (Tree Star Inc, USA). The positively stained apoptotic cells were counted, and the apoptotic index was calculated as the percentage of apoptotic cells within the total number of cells. Cells that stained only for Annexin V were considered early apoptotic (Annexin V+/PI-), and cells that dually stained for both Annexin V and PI were considered as late apoptosis (Annexin V+/PI+).
Data is presented as the mean ± SD, unless otherwise specified. Comparison of intergroup mean differences between the levels of studied markers in controls, AFB1-exposed, as well as treated groups, was performed using one-way analysis of variance (ANOVA). P values <0.05 were considered as significant. Statistical analysis of plasma markers was performed using GraphPad Prizm 5.0 software (GraphPad Software, Inc, USA).
First we evaluated the effect of Schiff bases on the studied parameters in intact animals (Schiff-base only group). In blood plasma (Figure 2A) and liver (Figure 2B) of the intact animals treatment with PLP (blood: p = 0.0023, liver: p = 0.0001), PLT (blood: p= 0.0002, liver: p=0.0342), and NLT (blood: p = 0.0001, liver: p = 0.0001) caused a significant decrease in MDA levels. No changes in SOD and CAT levels were observed during treatment with Schiff bases (Figure 3A and B), while a significant increase in total soluble AOA of blood serum was observed (Figure 4).
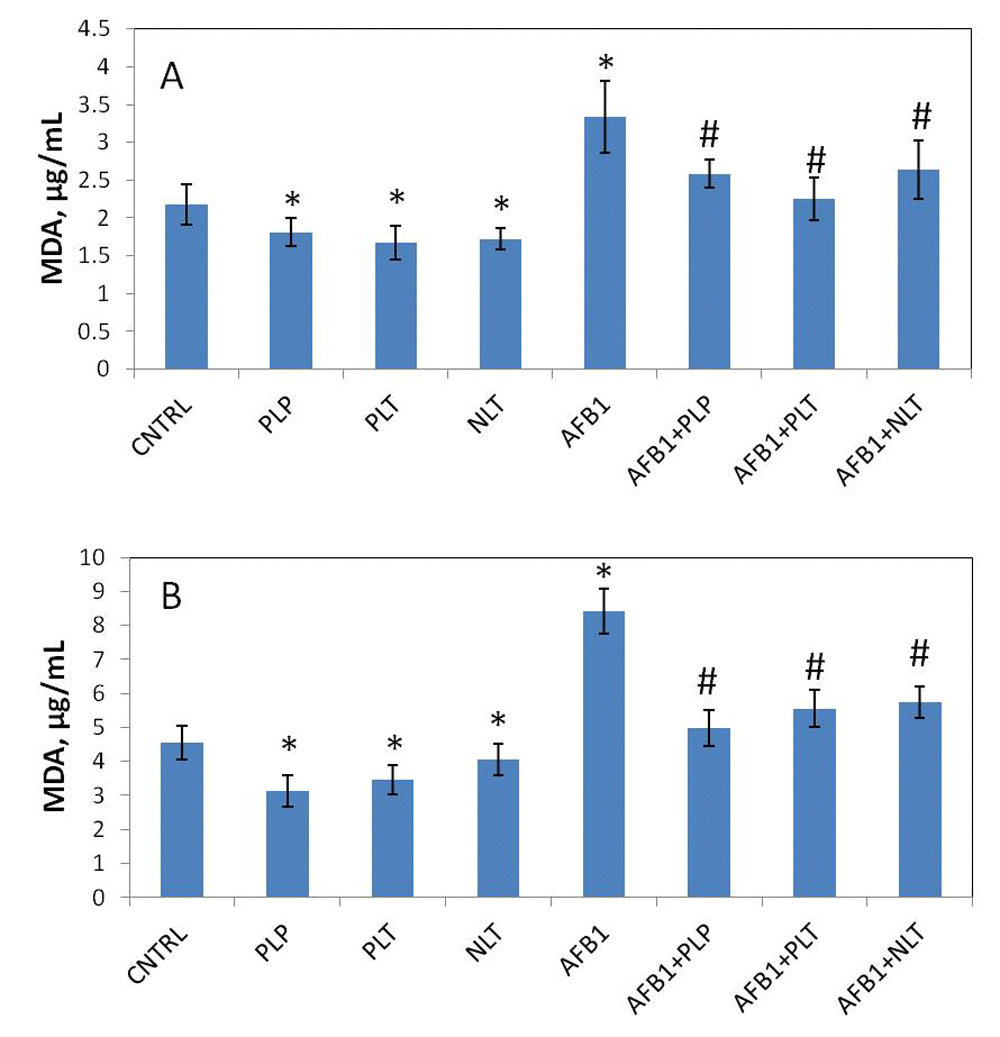
MDA levels in the plasma (A) and liver (B) of studied groups. CNTRL – intact animals (n = 10); PLP, PLT and NLT intact animals (n = 10 in each group) received 10-day oral treatment with corresponding Schiff bases at 10mg/kg dosage; AFB1 – rats (n = 10) treated with AFB1 for 21 days at 25μg/kg dosage; AFB1+PLP, AFB1+PLT, AFB1+NLT – AFB1 exposed rats (n = 10 in each group) treated with corresponding Schiff bases (mycotoxin and Schiff base dosages were similar in all treated groups). Data presented as mean±SD; *p<0.05 vs. CNTRL; #p< 0.05 vs. AFB1. AFB1, alflatoxin B1; MDA, malonic dialdehyde; PLP, picolinyl-L-phenylalaninate; PLT, picolinyl-L-tryptophanate; NLT, nicotinyl-L-tryptophanate.

Levels (µg/mL) of SOD (A) and CAT (B) in blood plasma of studied groups. CNTRL – intact animals (n = 10); PLP, PLT and NLT intact animals (for SOD: n =10 in each group; for CAT: n = 10 in PLP and PLT, n = 9 in NLT groups) received 10-day oral treatment with corresponding Schiff bases at 10mg/kg dosage; AFB1 – rats (n = 5) treated with AFB1 for 21 days at 25μg/kg dosage; AFB1+PLP, AFB1+PLT, AFB1+NLT – AFB1 exposed rats (n = 5 in each group) treated with corresponding Schiff bases (mycotoxin and Schiff base dosages were similar in all treated groups). Data presented as mean±SD. *p<0.05 vs. CNTRL; #p<0.05 vs. AFB1. AFB1, alflatoxin B1; SOD, superoxide dismutase; CAT, catalase; PLP, picolinyl-L-phenylalaninate; PLT, picolinyl-L-tryptophanate; NLT, nicotinyl-L-tryptophanate.
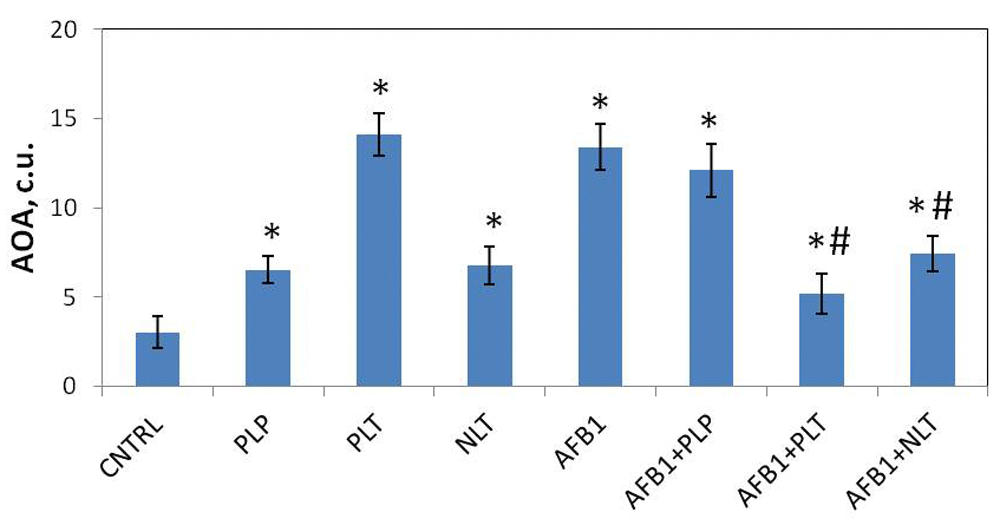
Total soluble antioxidant activity (expressed as conventional units, c.u.) in blood plasma of studied groups. CNTRL – intact animals; PLP, PLT and NLT intact animals received 10-day oral treatment with corresponding Schiff bases at 10mg/kg dosage; AFB1 – rats treated with AFB1 for 21 days at 25μg/kg dosage; AFB1+PLP, AFB1+PLT, AFB1+NLT – AFB1 exposed rats treated with corresponding Schiff bases (mycotoxin and Schiff base dosages were similar in all treated groups). Number of animals in all groups was 6. Data presented as mean±SD. *p<0.05 vs. CNTRL; #p<0.05 vs. AFB1. AFB1, alflatoxin B1; AOA, antioxidant activity; PLP, picolinyl-L-phenylalaninate; PLT, picolinyl-L-tryptophanate; NLT, nicotinyl-L-tryptophanate.
Next we analyzed the changes of the above mentioned parameters in AFB1 and AFB1+Schiff bases groups of animals. We assessed the intensity of AFB1-induced lipid peroxidation and the effects of the Schiff bases. According to our results, AFB1 induced a significant increase of MDA both in plasma (59%, p = 0.0001) and liver (85%, p = 0.0001) in rats of mycotoxicosis group (Figure 2), which suggests about activation of lipid peroxidation processes. Treatment of mycotoxicosis with Schiff bases caused a significant decrease of MDA levels both in blood and liver (Figure 2).
Next, we tested the status of enzymatic free radical defense system during AFB1 mycotoxicosis and Schiff base treatment 10 mg/kg dose level. The levels of SOD were significantly increased (p=0.005) in the plasma of AFB1 treated rats, while no difference was observed for CAT levels (Figure 3A). Treatment with PLT and NLT decreased SOD levels to its control values, while treatment with PLP reduced its levels further (p=0.001) (Figure 3A).
Finally, we observed fourfold elevation of total soluble AOA of blood serum non-enzymatic water-soluble antioxidants in AFB1 (p = 0.0001) mycotoxicosis compared to control. Treatment with PLT (p = 0.0001) and NLT (p = 0.0013) had a tendency to normalize AOA levels in ABF1-exposed animals, while PLP had no clear impact on their levels (Figure 4).
The increase of the malonic dialdehyde in blood plasma and liver homogenate suggests the intensification of lipid peroxidation in the organ and systemic levels. It is well known that AFB1 metabolizes into aflatoxin-8,9-epoxide, which aggressively interacts with DNA and forms adducts8,9. Moreover, in line with our results it has been shown that AFB1 induces lipid peroxidation in liver25. Meanwhile, we observed the increase of the levels of SOD and water-soluble non-enzymatic antioxidants, which can be a compensatory reaction to counterbalance oxidative stress. Schiff bases (PLT and NLT) were shown to normalize the levels of both SOD and AOA levels, as well as decrease the levels of MDA, which indicates their ability to interfere with the process of ROS generation in response to AFB1 exposure. Though the precise mechanism of their action is unknown, it was proposed that it might be related to the contents of active hydroxyl and amino groups of the Schiff bases26,27.
In order to evaluate the immune system changes caused by exposure to AFB1 and the effects of Schiff bases, the levels of terminal complement component (C5b-C9, TCC), circulating immune complexes, as well as the rates of early and late apoptosis of neutrophils and monocytes were assessed.
The results showed a statistically significant increase (p = 0.021) of TCC levels (marker of complement activation) and reduced (p = 0.003) levels of CICs in the blood plasma of AFB1-exposed animals (Figure 5). The treatment with NLT and PLP further increased the levels of TCC (p = 0.001 and p = 0.003 compared to controls, respectively), in the meantime normalizing CIC levels to that of controls. By contrast, treatment with PLT decreased the levels of TCC to the control levels without affecting low CIC levels. Neither CIC nor TCC levels were affected by the treatment with Schiff bases alone (Figure 5).
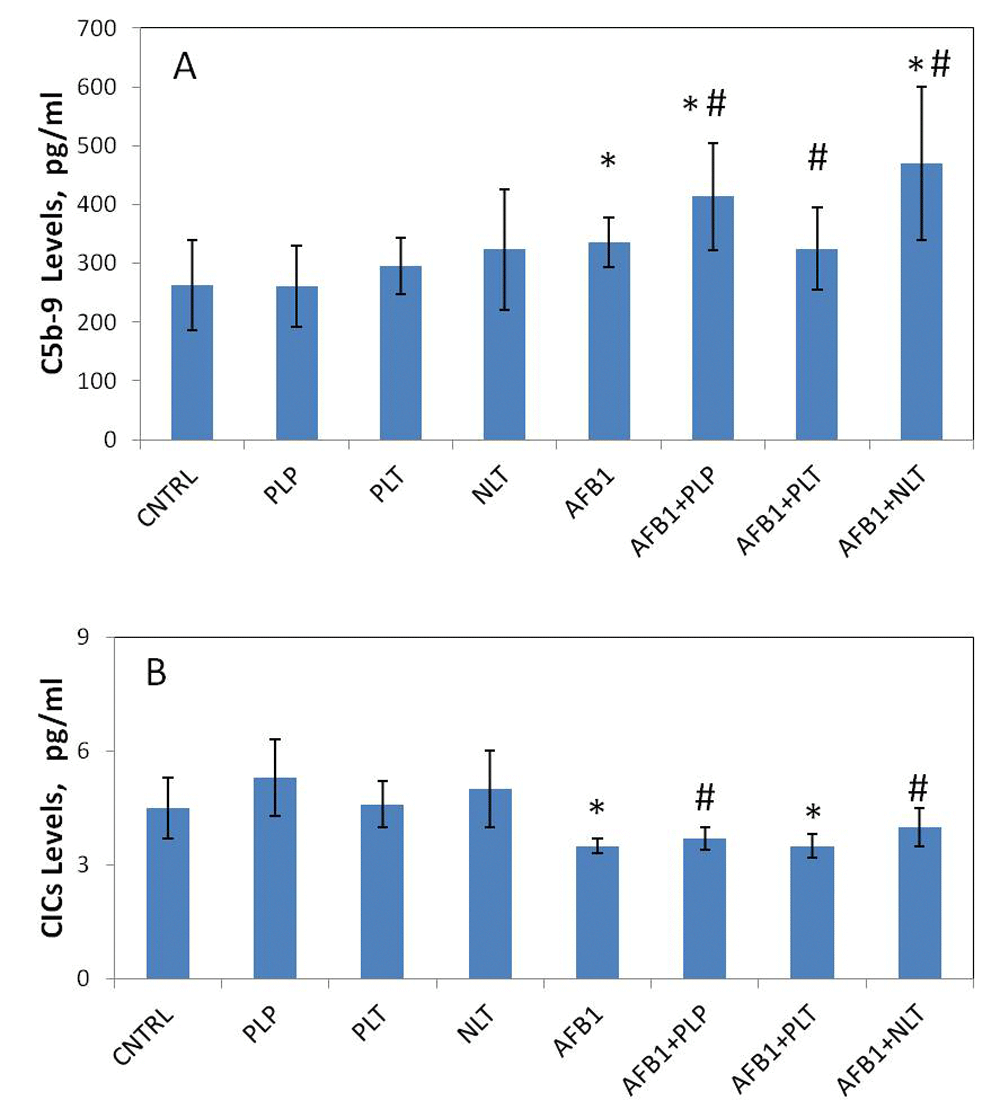
Levels (pg/mL) of terminal complement complexes (A) and CICs (B) in blood plasma of studied groups. CNTRL – intact animals (n = 10); PLP (n = 9), PLT (n = 8) and NLT (n = 10) intact animals received 10-day oral treatment with corresponding Schiff bases at 10mg/kg dosage; AFB1 – rats treated with AFB1 for 21 days at 25μg/kg dosage (n = 8); AFB1+PLP (n = 6), AFB1+PLT (n = 6), AFB1+NLT (n = 9) – AFB1 exposed rats treated with corresponding Schiff bases (mycotoxin and Schiff base dosages were similar in all treated groups). Data presented as mean±SD. *p<0.05 vs. CNTRL; #p<0.05 vs. AFB1. AFB1, alflatoxin B1; CICs, circulating immune complexes; PLP, picolinyl-L-phenylalaninate; PLT, picolinyl-L-tryptophanate; NLT, nicotinyl-L-tryptophanate.
The spontaneous apoptotic rates (early and late) of whole blood neutrophils and monocytes from control rats were not different compared with those from the rats treated with NLT, PLT, and PLP (Figure 5). The only exception was significantly reduced late apoptosis of neutrophils in the blood of rats treated with PLT (p = 0.032) and PLP (p = 0.034). AFB1 aflatoxin had a profound effect on the apoptosis of neutrophils and monocytes (Figure 6 and Figure 7). Particularly, in mycotoxocosis induced rats significantly accelerated early apoptosis of neutrophils and monocytes (p = 0.045) and late apoptosis of monocytes (p = 0.0027) were observed. Meanwhile, the treatment of AFB1-exposed rats with NLT significantly decreased the apoptotic rate of neutrophils (p = 0.05 and p = 0.036, respectively) and monocytes (p = 0.011 and p = 0.0025, respectively). We also observed a significant effect of PLT on the late apoptosis of neutrophils (p = 0.035) and monocytes (p = 0.0075) in the rats with developed aflatoxicosis compared to untreated rats. A less prominent effect was shown for PLP. The only significant difference was found in the late apoptosis of monocytes (p = 0.016).
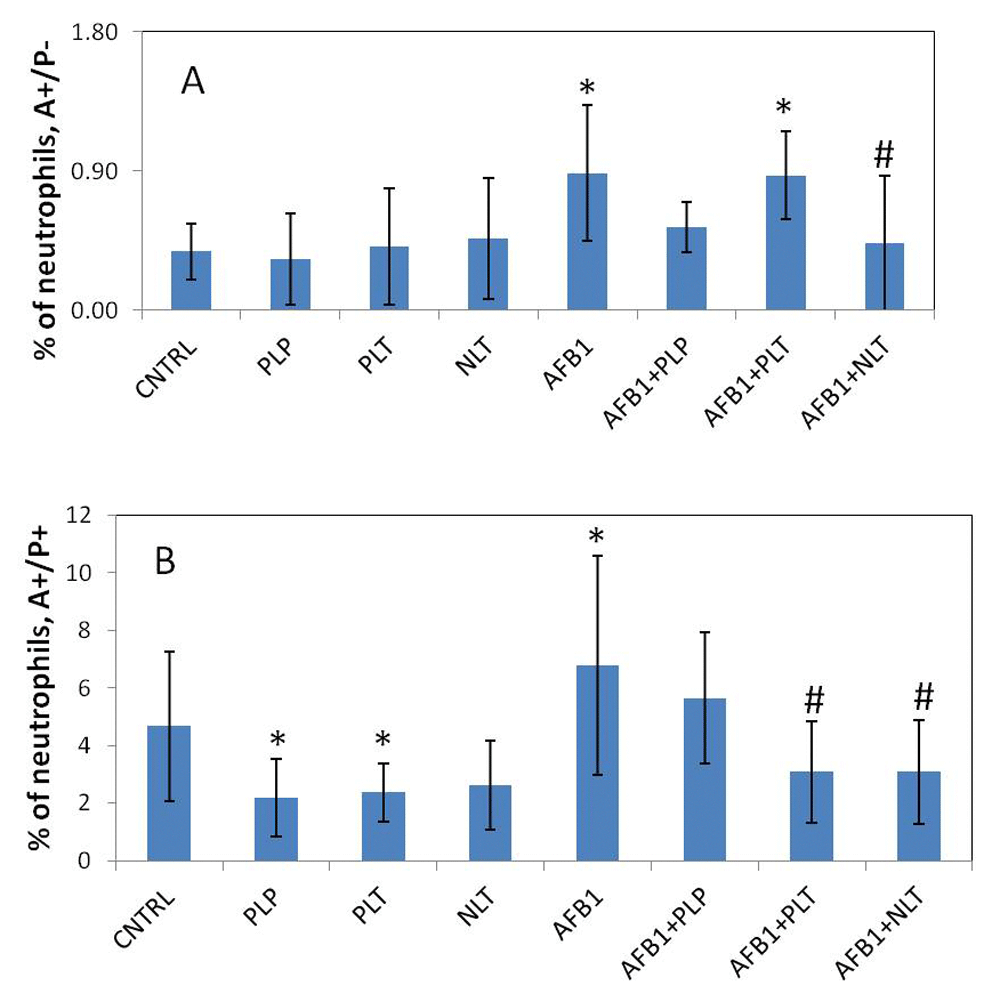
Spontaneous (A) and induced apoptosis (B) of neutrophils in studied groups. CNTRL – intact animals (n = 5); PLP, PLT and NLT intact animals received 10-day oral treatment with corresponding Schiff bases at 10mg/kg dosage (n = 9 in each group); AFB1 – rats treated with AFB1 for 21 days at 25μg/kg dosage (n = 9); AFB1+PLP, AFB1+PLT, AFB1+NLT – AFB1 exposed rats treated with corresponding Schiff bases (mycotoxin and Schiff base dosages were similar in all treated groups) (n = 9 in each group). Data presented as mean±SD. *p<0.05 vs. CNTRL; #p<0.05 vs. AFB1. AFB1, alflatoxin B1; PLP, picolinyl-L-phenylalaninate; PLT, picolinyl-L-tryptophanate; NLT, nicotinyl-L-tryptophanate.
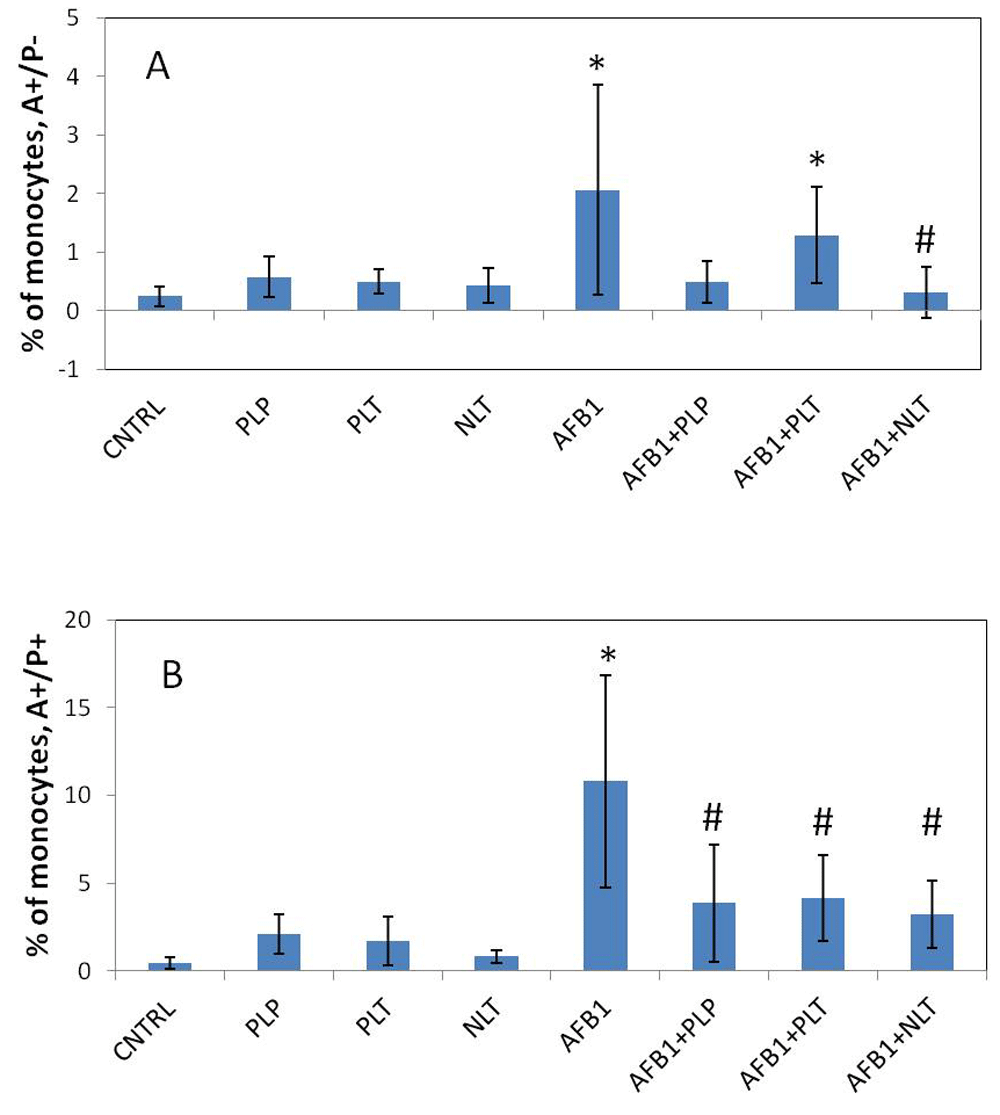
Spontaneous (A) and induced apoptosis (B) of monocytes in studied groups. CNTRL – intact animals (n = 5); PLP, PLT and NLT intact animals received 10-day oral treatment with corresponding Schiff bases at 10mg/kg dosage (n = 9 in each group); AFB1 – rats treated with AFB1 for 21 days at 25μg/kg dosage (n = 9); AFB1+PLP, AFB1+PLT, AFB1+NLT – AFB1 exposed rats treated with corresponding Schiff bases (mycotoxin and Schiff base dosages were similar in all treated groups) (n = 9 in each group). Data presented as mean±SD. *p<0.05 vs. CNTRL; #p<0.05 vs. AFB1. AFB1, alflatoxin B1; PLP, picolinyl-L-phenylalaninate; PLT, picolinyl-L-tryptophanate; NLT, nicotinyl-L-tryptophanate.
Mycotoxins may induce severe immunosuppression by downregulation of T and B lymphocyte activity, inhibition of antibody production and synthesis of complement components and interferon as well as impairment of macrophage-effectors cell function. While the exact mechanisms of mycotoxins action on immune system are presently unknown, oxidative stress, DNA damage, inhibition of gene expression and protein synthesis can be involved in immunosuppressive action of mycotoxins23,28,29.
In this study, we observed massive induction of apoptosis of neutrophils and monocytes induced by AFB1, which is in line with a previous report30. It is known that apoptotic cells activate complement. Subsequently, complement binding by apoptotic cells in normal human plasma occurs mainly to late apoptotic, secondary necrotic cells, and the dominant mechanism involves the classical pathway of complement activation by antibodies. Depletion of antibodies abolishes most complement fixation by apoptotic cells and causes delayed clearance of them31. Furthermore, we for the first time, reported the decrease of circulating immune complexes and the increase of circulating terminal complement component in AFB1-treated mice, which is in line with these findings. In this regard, Schiff bases (PLP and NLT) were shown to have modulating effect on immunity, by decreasing apoptosis rate and restoring the ability of efficient removal of apoptotic cells, which can be seen by restoring the TCC and CIC levels.
The results obtained in this study clearly demonstrated that AFB1 administration induced oxidative cell damage, immunosuppression and apoptosis of circulating immune cells. The oral administration of Schiff base cyclic amino acid derivatives is capable of minimizing the detrimental effects of mycotoxicosis by possessing multi-mechanistic effects that target AFB1-induced pathological events.
Dataset 1. Raw data for raw values for all the commercial ELISA kits (SOD, CAT, TCC and CIC), the values from the MDA, AOA. Dataset 1 contains tables with raw data for all measured assays except flow cytometry. CNTRL – intact animals; PLP, PLT and NLT intact animals received 10-day oral treatment with corresponding Schiff bases at 10mg/kg dosage; AFB1 – rats treated with AFB1 for 21 days at 25μg/kg dosage; AFB1+PLP, AFB1+PLT, AFB1+NLT – AFB1 exposed rats treated with corresponding Schiff bases (mycotoxin and Schiff base dosages were similar in all treated groups). Measurement units provided in corresponding table legend. doi, 10.5256/f1000research.11756.d16918032
FACS output files for neutrophils and monocytes are available at Zenodo33.
The authors acknowledge funding from the International Science and Technology Center (ISTC) in the frame of A-2116 (MM and AA) grant.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank Hakob Devejyan and Svetlana Kirakosyan for their advice and assistance in experiments.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Mehrzad J, Klein G, Kamphues J, Wolf P, et al.: In vitro effects of very low levels of aflatoxin B₁ on free radicals production and bactericidal activity of bovine blood neutrophils.Vet Immunol Immunopathol. 2011; 141 (1-2): 16-25 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology and single-immune cell technologies
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 10 Aug 17 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)