Keywords
fragility fracture, vitamin D, osteoporosis, bone health
fragility fracture, vitamin D, osteoporosis, bone health
Fragility fractures are defined by their low-energy nature, occurring from a fall or impact from a standing height or lower. They are the result of an underlying problem in the bone itself—for example, low density or abnormal remodeling—and, therefore, potentially preventable. Low bone mineral density (BMD) has been widely accepted as the target for treatment and prevention of these fractures, given its high prevalence and economic burden. Fifty-five million adults in the U.S. have low bone density, as defined by osteopenia or osteoporosis, sustain 1.5 million fragility fractures annually, and cost an estimated $22 billion annually for osteoporosis-related care1–2. Primary prevention is the ultimate goal; unfortunately, in clinical practice the diagnosis of low BMD is often not made until after fragility fractures have already occurred.
A key explanation for this diagnostic lag is the limitations BMD measurements have in predicting fragility fracture risk via existing methods, particularly dual-energy x-ray absorptiometry (DEXA). Measurements of BMD obtained by this test provide a snapshot of a patient’s bone density at a single time point, but bone metabolism is a dynamic process that is not fully reflected by BMD measurements alone. As many as 50% of patients with fragility fractures do not have osteoporosis as defined by their bone density3–5. Recognizing this limitation, the World Health Organization developed the Fracture Risk Assessment Tool (FRAX) to guide treatment and prevention strategies for fragility fractures. This online resource (https://www.sheffield.ac.uk/FRAX/) calculates 10-year fracture risk by integrating BMD with patient risk factors and is based on clinical data from a global cohort encompassing over 250,000 person-years of observation6–7. However, FRAX does not account for all patient factors that likely have an impact, and among these include fall risk, bone turnover markers, or certain medications. Moreover, FRAX has been shown to underestimate fracture risk in patients with diabetes, for example, as this particular risk factor is excluded from the calculation8.
In particular, FRAX does not account for serum 25-hydroxyvitamin-D [25(OH)D] levels. Vitamin D plays a key role in bone metabolism, and its supplementation is a mainstay in osteoporosis treatment and prevention, given its key role in bone metabolism9–12. Hypovitaminosis D is defined as a serum level <30 ng/ml and is present in nearly 70% of the U.S. adult population, according to National Health and Nutrition Examination Survey (NHANES) data13. Although the relationships between 25(OH)D levels and BMD, as well as that between BMD and fragility fractures, have been reported14–19, these previous studies focused primarily on hip and vertebral fragility fractures20–28. The relationship between 25(OH)D levels, fragility fractures, clinical risk factors, and BMD in a single cohort of referral patients has not been studied.
Recently, our orthopedic surgery department established a referral service for patients who have either sustained or been identified as being at risk for sustaining fragility fractures. Serum 25(OH)D levels, demographic data, clinical risk factor assessments, and BMD measurements using DEXA are routinely obtained for all patients at their initial office visit. The objective of this study is to evaluate the relationships between the data collected and the prevalence of fragility fractures in this cohort of referral patients. We hypothesized that both BMD measurements and serum 25(OH)D levels are lower in patients with a prior fragility fracture compared to those without.
Following approval by our institutional review board, the charts of patients presenting to our Bone Health Clinic were reviewed. Patients age 50 and older without a history of primary metabolic bone disorders (osteomalacia, Paget disease, or primary hyperparathyroidism) or oncologic bone disorders (primary or metastatic disease involving the skeleton) were included. Patients were included regardless of their vitamin D supplementation status prior to presentation. At the initial visit, each patient’s demographic data (age, race, and sex), body mass index (BMI), smoking history, corticosteroid use, and medical comorbidities (in particular, diabetes mellitus [DM] and rheumatoid arthritis [RA]) were recorded. A serum 25(OH)D level (ng/mL) was obtained for all patients. In addition, a DEXA scan to measure areal BMD T-scores at the lumbar spine, femoral neck, total hip, and distal radius (g/cm2) was obtained for those patients who had not had a DEXA within the six months prior to their first visit. The lowest BMD T-score value among the anatomical sites scanned was analyzed, consistent with our clinical practice preferences in guiding treatment decisions. Prior history of fragility fractures, if applicable, was documented in detail and included the mechanism of injury, date of injury, fracture site, and number of fractures sustained. (Dataset 1)
Discrete data, including patient demographics and risk factors, were described using means (SD) or proportions, as appropriate. Normality of the continuous variables, including 25(OH)D level, BMD measurements, BMI, and age, was assessed using QQ plots and boxplots. All categorical and continuous variables were compared between the fracture and no fracture groups using the Student t-test, chi-squared, or Fisher’s exact test, as appropriate. A subgroup analysis was also performed using these methods among the fracture patients to compare patients with a prior hip fracture to those with a prior fragility fracture elsewhere. Multivariate logistic regression analysis was performed including all covariates with a p-value less than 0.2 in the bivariate analyses, as well as 25(OH)D and BMD, to see whether these markers are independent predictors of referral group (i.e., prior occurrence of fragility fracture). Association of age, race, gender, BMI (and any other variables) with each of the 25-D and BMD measurements were performed with Pearson correlation or t-test as appropriate. A multivariate linear regression was also performed for each of BMD and 25-D level including all covariates with a p-value less than 0.2 in the above bivariate analyses to see whether these covariates are independently associated with 25-D or BMD. The Pearson correlations between BMD and 25(OH)D were computed within the two referral groups separately, as well as within the entire sample. For all analyses, a two-tailed p < 0.05 was considered significant. Bootstrap analysis and permutation tests were used to confirm bivariate results when there were concerns about normality of data.
One hundred and fourteen patients’ charts were identified. Twelve of these patients were excluded: ten lacked either a 25(OH)D level or DEXA scan available for review, and two had a diagnosis of primary hyperparathyroidism. Therefore, 102 patients were included in the study for analysis. 91 of these patients were women, and 11 were men. Sixty-four presented with a prior history of a fragility fracture (55 women, 9 men), while 38 of them presented without a prior history of a fragility fracture (36 women, 2 men). Mean patient age was 68, with a range of 50 to 92. Ninety patients were white, seven were black, and five were of other races. Average patient BMI was 28.71 and ranged from 15.14 to 45.58. The average 25(OH)D level was 37.66 ng/ml and ranged from 11 to 105 ng/ml. There were 12 patients with DM, seven patients with RA, four with a prior history of or current usage of corticosteroids, and 47 with a positive smoking history.
Prior fragility fractures occurred in 64 patients. The fractures recorded included six distal radius fractures, four fractures of the upper extremity other than distal radius, 15 hip fractures, 18 fractures of the lower extremity other than hip, 13 fractures of the thoracic spine, and 23 fractures of the lumbar spine. Fifty-one patients presented with one prior fragility fracture, 11 presented with two prior fragility fractures, and two presented with three. Eleven fragility fractures had occurred within one month prior to initial presentation, 28 occurred between one and six months prior, five occurred between six and 12 months prior, five occurred more than one year prior, and 15 occurred at an unknown prior time.
The fracture and non-fracture groups did not differ significantly with regard to sex, race, smoking history, steroid use, DM or RA. (Table 1) Additionally, the two groups did not differ significantly with regard to age, BMI, BMD value, or serum 25(OH)D level. (Table 2)
| Predictor | Mean (Y, N) | St Dev (Y, N) | P-value |
|---|---|---|---|
| Age | 69.48, 66.95 | 10.64, 9.46 | 0.215 |
| BMI | 28.08, 29.77 | 6.47, 6.47 | 0.206 |
| BMD T-score | -2.28, -1.82 | 1.33, 1.1 | 0.076 |
| 25(OH) level | 37.12, 38.55 | 17.02, 16.42 | 0.676 |
In the subset of patients with a prior fragility fracture (n=64), those who had sustained a hip fracture (n=15) had significantly lower BMD values compared to those who had sustained a fracture elsewhere (mean -3.12 vs. -2.03, p=0.004). There were no significant differences with regard to serum 25(OH)D level or any of the other variables measured when comparing these two subgroups. (Table 3 and Table 4)
| Variable | Hip Fracture Mean (SD) | Non-hip Fracture Mean (SD) | P-value |
|---|---|---|---|
| 25(OH)D | 34.33 (25.49) | 37.98 (13.69) | 0.602 |
| BMD T-score | -3.12 (1.02) | -2.03 (1.32) | 0.004 |
Sex, race, smoking history, DM, and steroid use were not significantly associated with measured serum 25(OH)D levels. However, patients with RA had significantly lower serum 25(OH)D levels upon presentation compared to non-RA patients (mean 22.57 vs. 38.77, p=0.001). (Table 5) Age and lowest BMD value were also not significantly associated with measured serum 25(OH)D level. However, BMI was negatively correlated with serum 25(OH)D level (R=-0.211, p=0.033). (Table 6, Figure 1)
| Variable | Pearson Correlation | Pearson P-value |
|---|---|---|
| Age | 0.151 | 0.129 |
| BMI | -0.211 | 0.033 |
| BMD T-score | 0.107 | 0.308 |
Sex, race, smoking history, DM, RA, and steroid use were not significantly associated with the lowest BMD measurement. (Table 7) However, age was negatively correlated with low BMD value (R=-0.269, p=0.009), whereas BMI was positively correlated with low BMD value (R=0.259, p=0.013). (Table 8, Figure 2 and Figure 3)
| Variable | Pearson Correlation | Pearson P-value |
|---|---|---|
| Age | -0.269 | 0.009 |
| BMI | 0.259 | 0.002 |
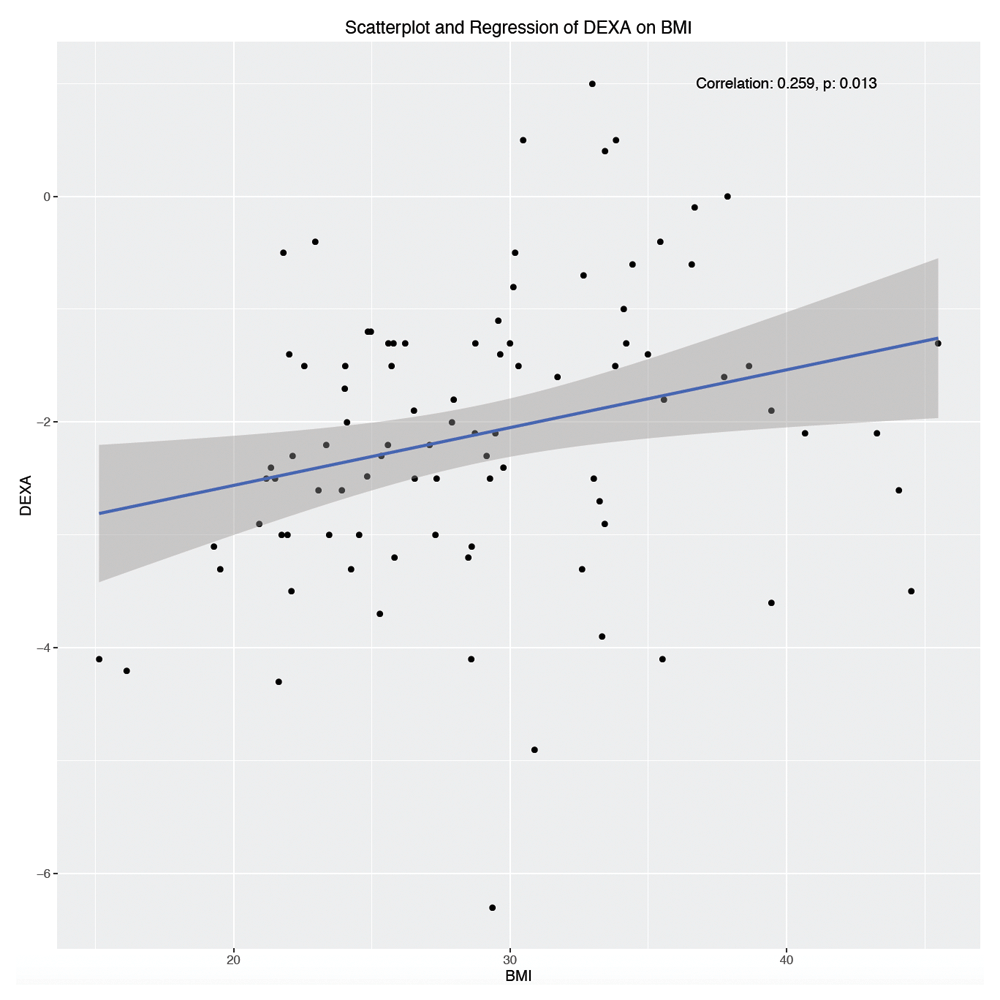
A scatterplot of patients’ lowest measured DEXA T-score as a function of their body mass index reveals a positive correlation (r=0.259, p=0.013).
Fracture liaison services are increasing in popularity as an adjunct to traditional orthopedic fracture care. These liaison services have been successful in providing more comprehensive medical evaluation and treatment for some fragility fracture patients29–32. Post-fracture osteoporosis care continues to be a treatment gap, since the majority of patients with fragility fractures are likely to “fall through the cracks” after their orthopedic care, without a proper referral system in place33–35. Our Bone Health Clinic aims for primary and secondary prevention of fragility fractures and, thereby, has expanded its indications for referral to also include patients who have yet to fracture prior to their initial visit.
Fracture and non-fracture groups did not differ with respect to any of the demographic variables evaluated. This suggests that our non-fracture patients were a suitable control group. Although our control group is valid on the basis of our interest in studying this specific referral population, this data is not generalizable to all adults age 50 and over in the U.S. This differs from previous studies which utilize publicly available data, such as NHANES, to estimate fragility fracture risk26,28.
In this study, serum 25(OH)D was not associated with fragility fractures. The literature regarding the predictive value of 25(OH)D levels in the setting of fragility fractures is conflicting, and this may be because most existing cohort studies analyze different fragility fracture sites. Swanson et al., Bakhtiyaroya et al., and LeBoff et al. studied fragility fractures of the hip and found lower 25(OH)D levels in patients with hip fractures as compared to controls24,25,27. However, Maier et al. studied vertebral fragility fractures and found no significant difference in 25(OH)D levels of patients admitted with vertebral fragility fractures compared to a control group23. Furthermore, Rozental et al. found no significant difference in 25(OH)D levels in patients who had sustained a fragility fracture of the distal radius as compared to controls36. Our study analyzed all fragility fracture sites, with the exception of our subgroup evaluation of 25(OH)D level in hip-fracture patients compared to non-hip-fracture patients. Our findings agree with prior studies and suggest that the predictive value of serum 25(OH)D levels may depend upon the specific fracture site. In a population study of nearly 5,000 patients, Looker found that 25(OH)D levels were a significant linear predictor of major osteoporotic fracture and a significant quadratic predictor of hip fracture. However, in the same study 25(OH)D levels did not predict fractures beyond 10 years after presentation26. This further supports the notion that fragility fracture sites differ with respect to the predictive value of 25(OH)D, with hip fractures having the strongest association. Further cohort studies with greater numbers are needed to distinguish the utility of 25(OH) among the various fracture sites.
The literature regarding the predictive value of BMD is also conflicting. Melton et al. found that low BMD measurements predicted fragility fractures at the hip and lumbar spine at long-term follow-up of ten years18. However, Schuit et al. found that less than half of the patients in their cohort with non-vertebral fractures had low BMD5. Furthermore, Marshall et al. performed a meta-analysis of 11 studies evaluating the relative risk for fracture with decreases in BMD and concluded that although BMD can be useful to predict fracture risk on a population level, it cannot predict which individuals will fracture37. Our findings confirm this data, as we found that BMD measurements are not associated with fragility fractures as a whole, except in patients sustaining hip fractures compared to those with fractures elsewhere. One potential explanation for the lack of fracture predictability using BMD in this study is that, for those patients with fragility fractures, the lowest DEXA value measured was not necessarily in the fractured area. Bone strength is derived not only from bone quantity but also bone quality, which consists of structure (micro- and macroarchitecture), turnover, and material properties—all of which are not assessed with DEXA. Our finding that BMD decreases with age is also consistent with existing literature38–40.
In our study, all patients were considered to have sustained a fragility fracture if the fracture occurred after a fall from standing height or lower. It is possible that any high-energy fractures sustained by these patients previously, and which were excluded in our calculations using this definition, could have actually resulted in fragility fractures had the mechanism of injury been low energy. It has been reported that the exclusion of high-energy fractures underestimates the prevalence of fragility fractures in the community, and as a result BMD measurements have been recommended following all trauma in older adults regardless of the nature of the energy41. This knowledge, along with the findings of a previous study which found a high prevalence of hypovitaminosis D among patients admitted to an orthopedic trauma service42, suggests that older adult patients warrant post-fracture care work-up regardless of the mechanism of injury.
With regard to the clinical risk factors we studied, patients with RA had lower serum 25(OH)D levels. This confirms existing data which has well established the linkage between RA disease activity and severity, and decreased vitamin D synthesis43,44. However, we cannot conclude whether these patients’ 25(OH)D levels were the result of their disease.
The effect BMI has on fracture risk is difficult to determine based on our data, as we found a positive association of BMI with BMD and a negative association with 25(OH)D levels. Ekwaru et al. determined that the serum 25(OH)D level significantly drops as BMI increases among individuals with vitamin D supplementation45. We speculate that the concentration gradient of 25(OH)D may shift from serum to fat stores as BMI increases. On the other hand, the increased bone density afforded by being overweight may simply be due to the increased response of bone to mechanical stress, according to Wolff’s Law, as has been shown clinically46. Both of these associations with BMI were weak, so it is unclear how significantly BMI affects each of these markers. We conclude that obesity may confound the overall assessment of fracture risk in these patients.
Limitations of the present study include its retrospective nature as well as a small, homogeneous sample which is underpowered to detect the difference in 25(OH)D levels or DEXA values between the fracture and non-fracture groups. Given the reported high prevalence of hypovitaminosis D in the U.S. population, we were surprised to find that many of the patients studied had normal serum 25(OH)D levels. This is likely the result of supplementation by patients prior to referral. Future studies including a larger number of patients, in particular those who have not received prior vitamin D supplementation or other pharmacologic treatment are needed. While 25(OH)D levels may be of value in predicting fracture risk in treatment-naive patients, in those already on supplementation we found that its utility is largely limited to serving as a reference for dosing.
Our goal with this study to provide preliminary data on a referral population of patients deemed to have poor bone health will prove useful in the establishment of similar programs elsewhere, in light of the increasing trend in healthcare toward quality and outcomes measures. We conclude that the initial assessment of patients presenting to Bone Health Clinic is complex and requires a holistic approach, taking into consideration a multitude of factors.
Dataset 1: Demographic Data, Fracture Status, and Serum 25-Hydroxy-Vitamin D Levels for the Bone Health Clinic Patient Cohort
Each row represents a single patient’s information which is separated into columns based on topic of interest. These include age, sex, race, body mass index (BMI), fracture status on initial presentation, anatomical location of fracture(s) (if applicable), whether a patient with a prior fracture had sustained a hip fracture or multiple fractures, the time from most recent fracture sustained, DEXA T-scores included in separate columns with the lowest of the three listed in a separate column, serum 25(OH)D level, serum parathyroid hormone (PTH) level, rheumatoid arthritis status, smoking status, diabetes mellitus, and corticosteroid use. Y=yes, N=no. 10.5256/f1000research.12484.d175581
We would like to acknowledge Randal Morris who assisted with institutional review board (IRB) submission, coordination of data collection, and manuscript preparation. We also acknowledge Suzanne Simpson who assisted with copyediting of manuscript
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Osteoporosis epidemiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Endocrinology and metabolic bone diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 29 Aug 17 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)