Keywords
Isopoda, cryptic species, Sarasota, Crustacea, Ligia exotica
Isopoda, cryptic species, Sarasota, Crustacea, Ligia exotica
The isopod genus Ligia includes ~40 nominal species, most of which inhabit a narrow range in the upper rocky intertidal habitats. In the Greater Caribbean Region (i.e. the Caribbean and adjacent regions), a single endemic species is currently considered valid: Ligia baudiniana1,2. The species has been reported from Brazil3, the Caribbean islands4, the Pacific coastlines of Central and South America5–7, Bermuda8, Bahamas4, and in southern Florida9,10 and the Everglades4; however, doubt over historical records have left the distributional limits of L. baudiniana unclear.
L. baudiniana was described from specimens collected in the San Juan de Ulua Fort in Veracruz, Mexico. Milne-Edwards’ original species description11 focuses on characters that are of limited taxonomic importance12, lacks illustrations, and does not provide an account of male reproductive structures now known to be useful in Ligia taxonomy12–14. Indeed, the terse description and source origin of the type material (i.e., artificial substrate) have led to confusion on whether L. baudiniana is a synonym of L. exotica or a valid species15,16, and to records and specimens identified as L. baudiniana to be re-classified as L. exotica (3 and references). This is particularly true for specimens from the American Gulf of Mexico coastlines, as most records appear to have been reclassified as L. exotica. Furthermore, a wide-ranging survey of Ligia in the Gulf of Mexico from Texas to Florida has shown artificial habitats in the region to harbor only L. exotica (unpublished study; Hurtado LA, Mateos M, Wang C, Santamaria CA, Jung J, Khalaji-Pirbalouty V, and Kim W).
The taxonomic confusion between L. baudiniana and L. exotica is complicated by the presence of a Ligia species endemic to habitats throughout the Greater Caribbean, Gulf of Mexico excluded, that is easily recognized by a unique male gonopod morphology that is readily distinguishable from L. exotica (Figure 1), and that has been attributed to L. baudiniana by Andersson5, Rouse9, Schultz4,8, and Schultz and Johnson10. A recent molecular study demonstrated that Ligia exhibiting this trait form a well-supported monophyletic clade composed of several cryptic and highly divergent lineages endemic to the region14. The combination of these studies suggests that L. baudiniana as currently recognized: (a) is an endemic species to the Greater Caribbean Region; (b) can be identified using both molecular and morphological tools; and (c) appears to have a broad geographic range that includes the Caribbean islands, the Pacific coastlines of Central America to Ecuador, Bermuda, Bahamas, and southern Florida.
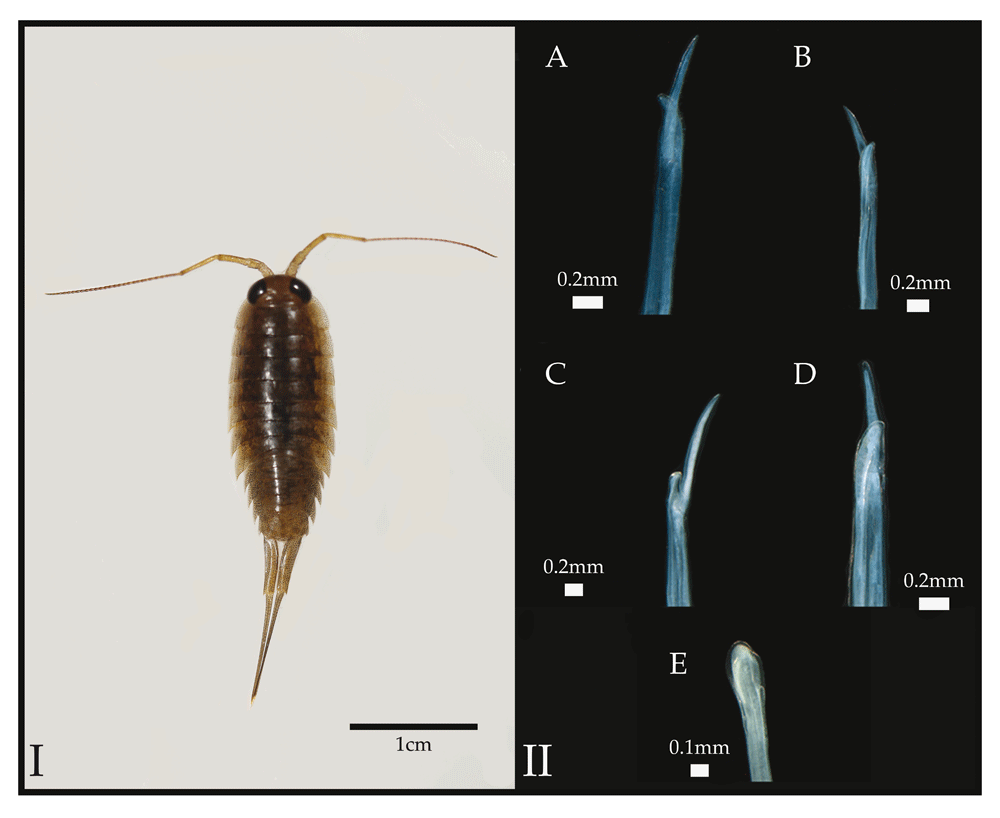
This trait was used to putatively identify specimens collected in this study. The appendix masculine of L. exotica is shown in II-E. Photographs in panel II are reproduced under a Creative Commons license from Santamaria et al. (2014)14.
In southern Florida, L. baudiniana is reported from the Florida Keys9,10 and the Everglades4, while no confirmed records from the American Gulf of Mexico exist to date. In this study, we use molecular and morphological approaches to identify specimens collected from Sarasota and Manatee counties in Florida as L. baudiniana. Our findings extend the confirmed range of this species ~300-km into the Gulf of Mexico coastline of Florida and represent the first confirmed record of L. baudiniana in the American Gulf of Mexico coastline.
Ligia specimens were collected by hand across the Sarasota-Manatee counties in Florida (Table 1, Figure 2) and field preserved in 70% EtOH. No permits were necessary for collections. Specimens were identified to species by inspecting the morphology of the apex of the endopod of the second pleopod of 15–25 male Ligia specimens per site, with individuals putatively identified as L. baudiniana if they exhibited a large process bifurcating close to the apex of the appendix masculina (Figure 1A), as proposed by Schultz4,8 and confirmed by Santamaria et al.14. A subset of specimens was deposited in the Invertebrate Collections of the Biodiversity Research and Teaching Collections (BRTC) at Texas A&M University (http://brtc.tamu.edu/).
New records are in bold.
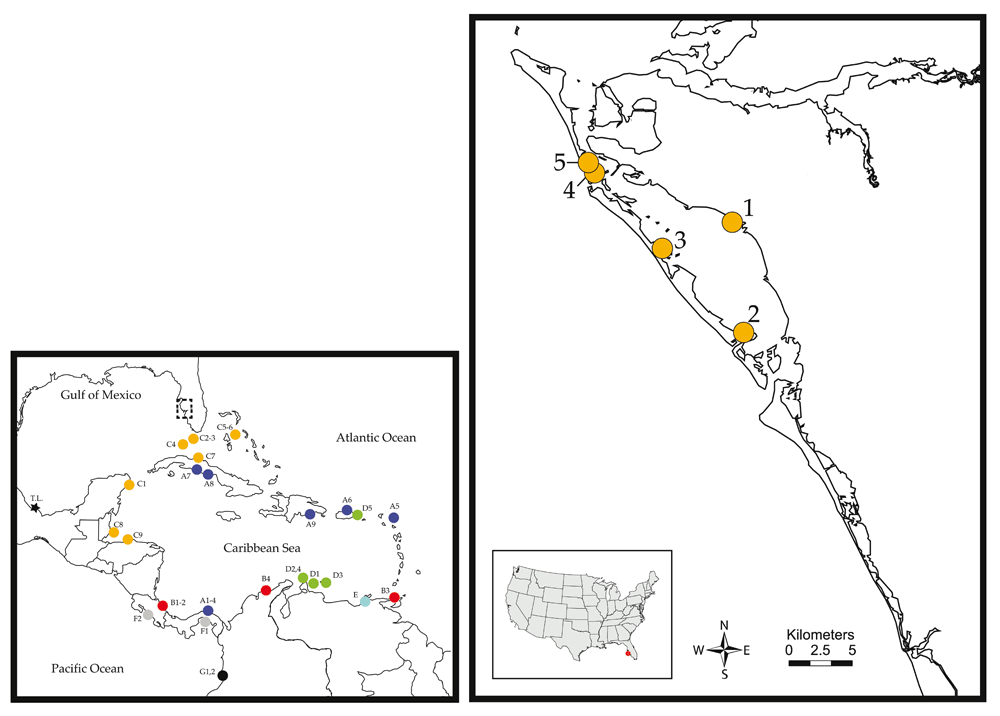
Locations are: (SRQ1) End of Tiara Drive; (SRQ2) Quick Point; (SRQ3) Joan M. Durante Community Park; (SRQ4) Leffis Key; (SRQ5) Near Coquina Beach. Detailed locality information can be found in Table 1. The smaller panel presents the distribution of L. baudiniana lineages reported to date throughout the Caribbean and its adjacent region.
Morphological identifications were corroborated using a mitochondrial barcoding approach. We extracted total genomic DNA from pleopods/pereopods for a subset of individuals putatively identified as L. baudiniana using the ZR Quick-gDNA Miniprep Kit. Previously described primers and conditions were used to PCR-amplify and sequence a 658-bp fragment of the Cytochrome Oxidase I gene (COI, primers LCO1490/HCO2198; 17). Positive amplicons were cleaned and sequenced at the University of Arizona Genetics Core (UAGC). Sequences were assembled in Geneious R v8.1.7.
We combined nucleotide sequences produced in this study with publicly available ones for L. baudiniana and L. exotica (Table 1). We used default settings to align the resulting dataset using the MUSCLE Alignment18 tool in Geneious R v8.1.7. No signs of misaligned regions or pseudo-genes were observed in the resulting alignment. The final alignment was imported into MEGA v7.0.1819, where we estimated a neighbor-joining tree under Kimura’s 2-parameter model (hereafter K2P; 20) and uniform rates. Support for the relationships within the tree were estimated by conducting 1,000 bootstrap replicates. Lastly, we calculated K2P genetic distances between haplotypes produced by this study, L. exotica, and previously reported L. baudiniana clades14.
Molecular identifications produced results congruent with morphological identifications. We obtained 12 unique COI haplotypes from a total of 25 individuals putatively identified as L. baudiniana. Haplotypes produced in this study were highly similar to each other (COI K2P 0.00–2.81%, Table 2) and to those reported from localities in the Florida Keys, The Bahamas, northern Cuba, Cozumel, and Bermuda (COI K2P 0.50–6.08%, Table 2). Haplotypes were moderately to highly divergent from L. baudiniana from other localities in the Caribbean (COI K2P 14.44–24.90%, Table 2), and highly divergent from L. exotica (COI K2P 20.32–25.18%). The neighbor-joining analysis produced similar results (Figure 3), nesting all haplotypes produced in this study in a well-supported clade (Bootstrap Support = 100) with the Clade C reported by Santamaria et al.14. All unique haplotypes have been deposited in GenBank (Table 1).
The top diagonals show minimum and maximum divergences between lineages, with lower diagonals presenting average genetic distances between clades. Within-group divergences are shown in the middle diagonal (in bold) in the order: minimum, maximum, and average divergence.
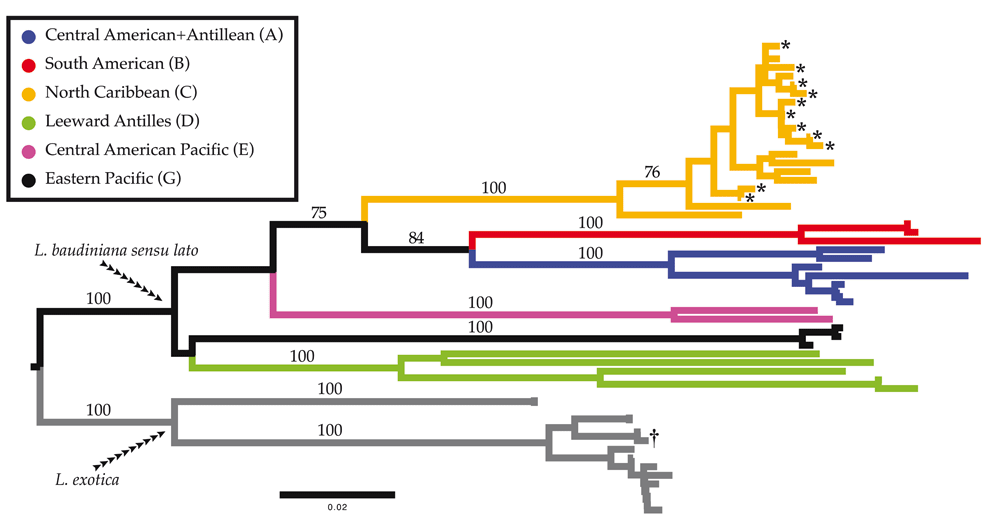
Molecular identifications of putative L. baudiniana samples from Sarasota were made using K2P distances. All haplotypes for Ligia from Sarasota-Manatee counties (denoted by an *) are placed with previously reported haplotypes from the North Caribbean Clade reported by Santamaria et al.14 in a well-supported clade (values near nodes represent bootstrap support values). Branches are drawn to scale, with colors and labels corresponding with those used by Santamaria et al.14. The COI haplotype obtained from topotypes of L. baudiniana by Santamaria et al.14 is denoted by a †.
Morphological and molecular evidence confirm that our sampled individuals represent L. baudiniana. These new records represent the first confirmed presence of this species in the Gulf of Mexico coastlines of the USA and extend the recognized range of the species ~300 km northward from a previous confirmed record from Florida Bay. Positive identifications in this study were made using both morphological and molecular characters. These findings are important as Florida’s rich coastal biodiversity faces serious threats such as sea-level rise, introduction of alien species, urbanization, habitat loss, and species displacements21.
All L. baudiniana specimens collected in our surveys were found in coastal mangrove forests with no specimens found in >10 surveyed artificial habitats. This suggests that coastal development in the American Gulf of Mexico may have led to the replacement of a native species with an introduced one via the removal of mangrove habitats for the establishment of artificial substrates. Additional work is needed to establish whether L. baudiniana is present in other mangrove habitats along the Gulf of Mexico, thus clarifying the northern limits of this species’ range.
We would like to acknowledge Taylor Greenan, Kathleen Newell, and Sharla C. Rafferty for their help in the field and the laboratory. We would also like to thank the University of South Florida Sarasota-Manatee for funding and access to shared facilities.
Supplementary File 1: Alignment of COI gene sequences for all sequenced individuals and L. baudiniana and L. exotica sequences from GenBank.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Marine Biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 30 Aug 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)