Keywords
Cerebral autoregulation, NIRS, Oximetry,
Cerebral autoregulation, NIRS, Oximetry,
Near-infrared spectroscopy (NIRS) has been adopted in clinical practice for over three decades now. Based on a similar principle as pulse oximetry, it allows continuous, non-invasive, real-time monitoring of cerebral oxygen saturation (ScO2) in a small sample of the frontal cortex. It was initially promoted as a brain monitor in cardiac surgery and neonatal intensive care, but its use has been extended to various non-cardiac surgeries and critical care settings. Some recent reviews provide excellent detail on the principles of NIRS and its current clinical practice patterns1–3.
The present review focuses on an emerging aspect of monitoring abilities with NIRS, which, when combined with continuous blood pressure monitoring, might prove to be a major step forward in critical care management: cerebral autoregulation monitoring.
Currently, blood pressure targets during perioperative and critical care management are mostly empirically chosen and rather fixed in any individual patient. This practice finds its origin in the concept of cerebral autoregulation, which refers to a modulating mechanism that controls cerebral blood flow (CBF) during changes in cerebral perfusion pressure (CPP). The classic curve of cerebral autoregulation represents the autoregulation plateau and the lower and upper limit of autoregulation (LLA and ULA, respectively) (Figure 1).
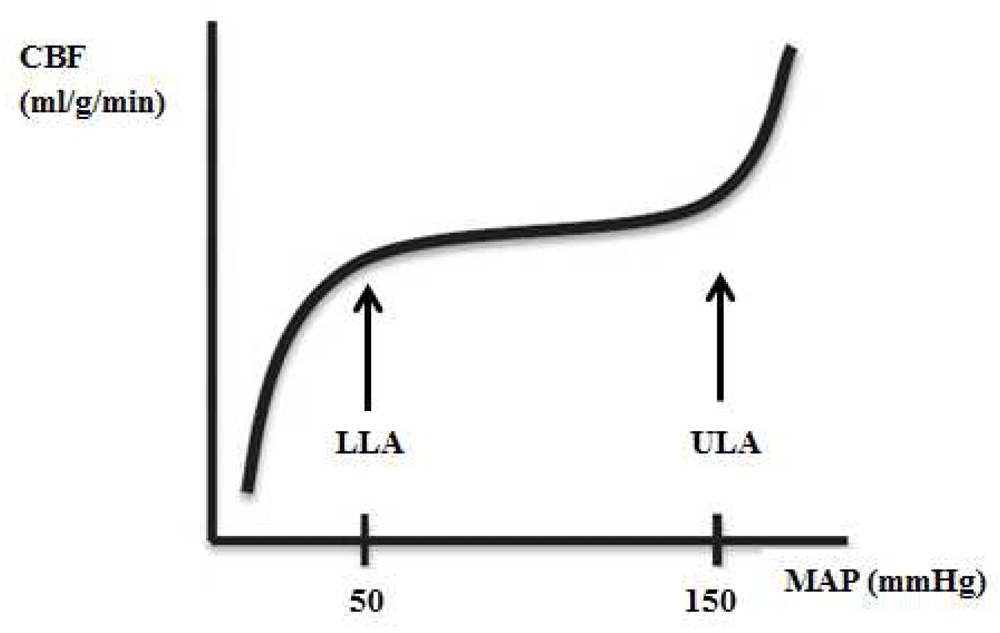
CBF, cerebral blood flow; MAP, mean arterial blood pressure.
Using data from 11 human studies, derived from young patients with few comorbidities, Lassen described an autoregulatory plateau between mean arterial blood pressures (MAPs) of 50 and 150 mm Hg, over which CBF remained constant by active myogenic control of small cerebral arteries and arterioles4. At the extremes of blood pressure (that is, below the LLA and above the ULA), myogenic vasoactivity capacity is no longer able to accommodate these changes, and CBF becomes pressure-passive; CBF decreases when MAP moves below the LLA and increases when MAP is above the ULA.
This description of cerebral autoregulation has been widely accepted and has been propagated in many textbooks, and, even today, most caregivers feel confident with a MAP within the presumed autoregulation range. However, already in 1997, the validity of this concept was criticized5. It is now accepted that CBF regulation is determined by many more factors than blood pressure alone. A variety of physiological and biochemical mechanisms may interact and lead to a complex entity of vascular reactivity in which the relative regulatory role of each component remains largely unknown6,7. It has also been acknowledged that autoregulation may be altered in specific diseases (for example, prematurity, hypertension, diabetes, and stroke) and in specific conditions (for example, changes in carbon dioxide and pharmacologic interference)6–12. Figure 2 gives an example from one of our own studies13. In this study, we managed hypertension with one of three different vasodilating drugs: sevoflurane, sodium nitroprusside (SNP), or nitroglycerin. Figure 2 clearly shows that, with sevoflurane and SNP, changes in MAP are highly correlated with changes in ScO2, suggesting impairment of cerebral autoregulation.
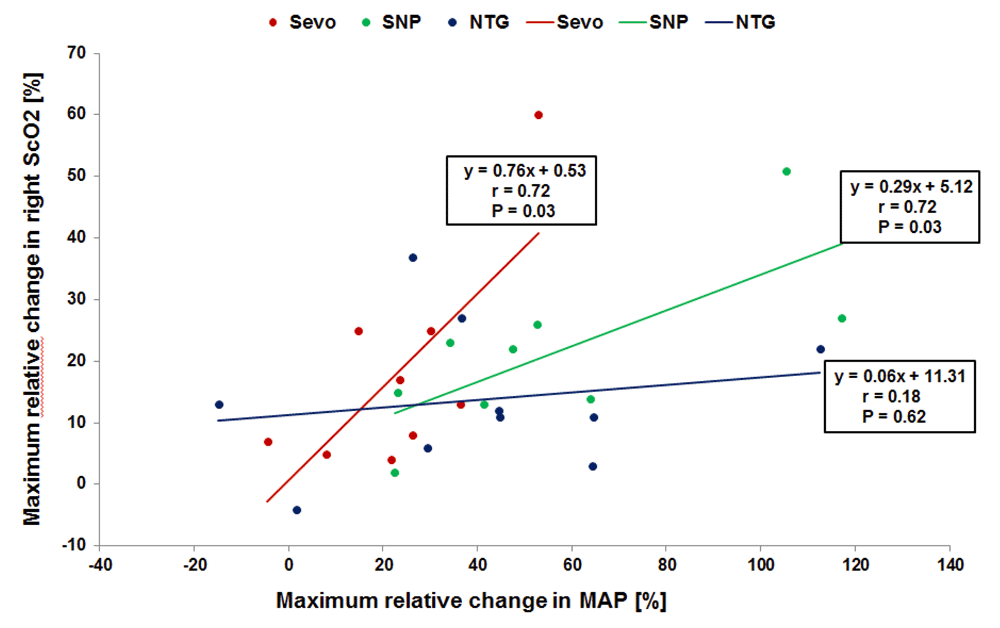
The drugs are sevoflurane (Sevo), sodium nitroprusside (SNP), and nitroglycerin (NTG). Own data, not published.
In response to all of these confounders affecting cerebral vascular reactivity, the limits of autoregulation and the shape of the autoregulation curve may vary enormously and unpredictably (Figure 3)14,15. Therefore, the practice of applying a fixed blood pressure threshold in an individual patient is risky, and tighter pressure control is mandatory16.
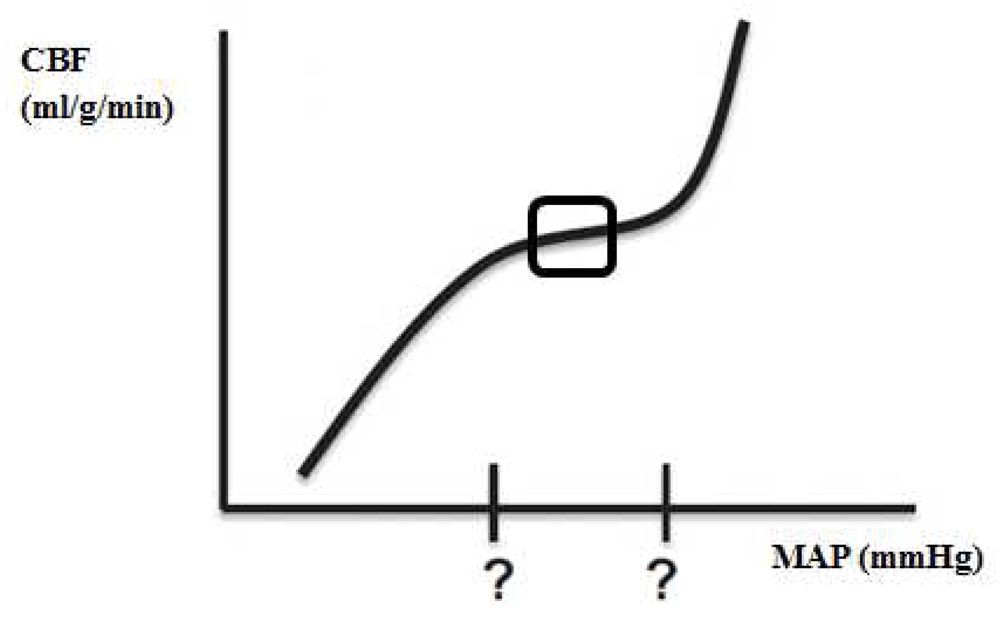
CBF, cerebral blood flow; MAP, mean arterial blood pressure.
Even with an impaired autoregulation curve, there might be an optimal blood pressure in the middle of the autoregulation curve at which the autoregulatory function is most robust (Figure 3, indicated with a square). Identifying the range of optimal cerebral autoregulation offers the potential for individualization of blood pressure targets, and integrating the ‘optimal MAP’ in daily critical care management might shift the current clinical paradigm of ‘one size fits all’ care to individualized, patient-specific, physiology-based blood pressure management.
To assess cerebral autoregulation, changes in CBF are plotted over a wide range of blood pressures. From a practical point of view, this requires a continuous measurement of arterial blood pressure (ABP) and a real-time estimate of CBF. Although theoretically CPP should be plotted, this requires invasive monitoring of intracranial pressure (ICP) (CPP = MAP − ICP). Therefore, without access to ICP monitoring and without an increased ICP, ABP is considered a valid substitute for CPP.
For the assessment of CBF, no convenient means of measuring CBF currently exist, so surrogates of CBF are being used. Recently, the various indices of autoregulatory function were extensively examined in adults with traumatic brain injury (TBI)17.
NIRS-measured ScO2 is considered a reliable surrogate of CBF. When ScO2 is correlated with MAP, an index of autoregulatory vasoreactivity, the cerebral oximetry index (COx), is generated. Blood pressure in the autoregulation range is indicated by a COx that approaches zero; that is, there is no correlation between ScO2 and MAP. A COx approaching one indicates a strong correlation between the two signals and has to be interpreted either as impaired autoregulation or as MAP being beyond the limits of autoregulation (Figure 4). The COx has been validated and had good agreement with transcranial Doppler-derived measurements of pressure autoregulation in piglets18 and in adult patients19–21.
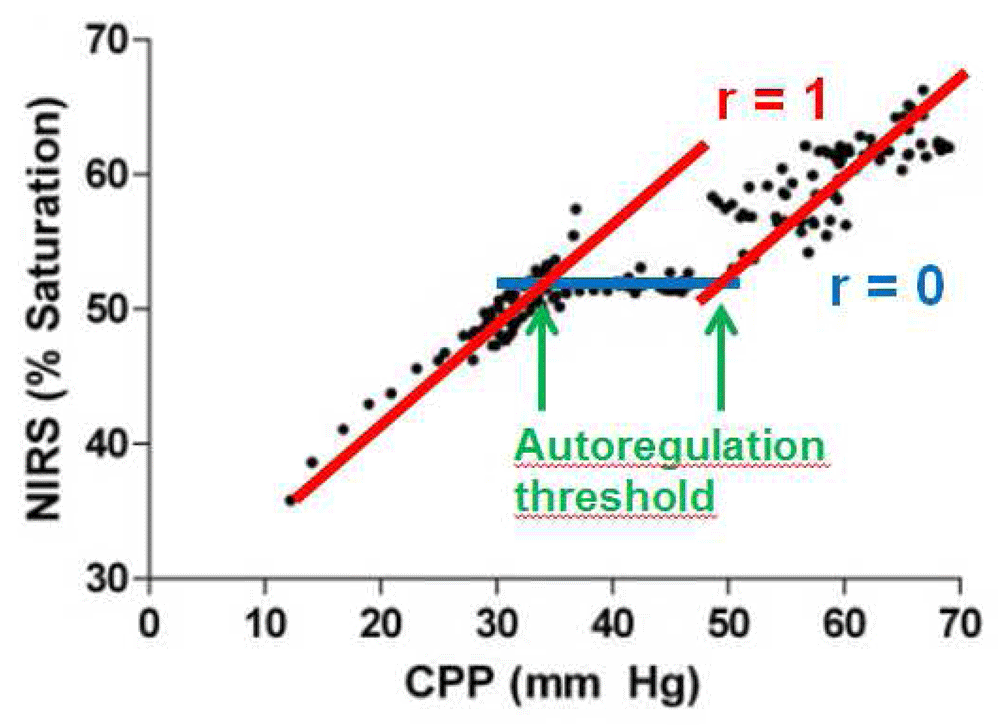
The correlations generate the cerebral oximetry index (COx). Adapted from Brady et al.18.
Studies which evaluate cerebral autoregulation with the use of NIRS have been expanding over the past few years. Owing to the high incidence of brain injury in these domains, research on this topic has been established mainly in the areas of neonatology, cardiac surgery, and neurocritical care.
NIRS was originally introduced in clinical practice in 1985 for the assessment of cerebral oxygenation in preterm infants22. Organ immaturity makes neonates vulnerable to physiological disturbances, and especially the brain may be at greater risk because of the incompletely developed cerebral autoregulation mechanism23,24. The need for firm hemodynamic boundaries has challenged continuous research in this area. We refer the interested reader to some recent reviews on this topic9,25,26.
In cardiac surgery patients, an ABP of 50–60 mmHg is widely considered to be an acceptable perfusion pressure during cardiopulmonary bypass (CPB). However, this practice was challenged by data showing a lower incidence of cardiac and neurologic complications when targeting a higher MAP (>80 mmHg) during CPB27. Impaired cerebral autoregulation has been demonstrated in 35% of cardiac surgery patients12, with a wide variability of the MAP at the LLA from 40 to 90 mmHg14. Consequently, in the case of empirically chosen MAP targets, patients may spend varying portions of time with MAP below the LLA during CPB.
In some recent studies, it has been demonstrated that MAP variations outside the autoregulatory range, not the absolute MAP values, were associated with adverse outcomes28–31. The time and magnitude that MAP spent below the LLA were higher for patients who developed acute kidney injury compared with patients without acute kidney injury, although absolute blood pressure values were equal between the two groups28. A significant association was also found between blood pressure excursions below the LLA and major morbidity (for example, renal failure, duration of mechanical lung ventilation, and low cardiac output syndrome)29. A recent study demonstrated that a blood pressure below the optimal MAP based on COx monitoring was associated with an increase in postoperative plasma GFAP (glial fibrillary acidic protein), which is a brain-specific injury biomarker30. However, on the other hand, simply raising blood pressure might result in a MAP above the ULA, which also predisposes to cerebral injury. Hori et al. demonstrated that the sum of the product of the magnitude and duration of MAP above the ULA was associated with increased risk for delirium in cardiac surgery patients31.
Patients with brain injuries constitute another population at high risk of impaired autoregulation. A consensus statement from the Neurocritical Care Society and the European Society of Intensive Care Medicine suggested that continuous monitoring of cerebral autoregulation might help guide ABP and CPP targets to ‘optimal’ levels in patients with TBI and might contribute to prognostication32. Management at or close to the optimal CPP has been shown to be associated with better outcomes in patients with TBI33,34. Since an in-depth discussion of cerebrovascular autoregulation monitoring in neurocritical care is beyond the scope of this article, we refer the interested reader to some excellent reviews6,35–39.
One methodological aspect of autoregulation monitoring with NIRS in the setting of neurosurgery and neurointensive care requires close attention. NIRS technology is based on sending near-infrared light through the tissues, where it is attenuated because of a combination of absorption and scattering. It is a prerequisite that the quantity of light scattering remains constant during the measurements and that changes in attenuation result solely from changes in absorption40. However, in conditions of brain injury, tissue composition may vary substantially (contusions, hemorrhages, and brain swelling), resulting in changes in light absorption and scattering. Accurate quantification of ScO2 and data quality could be questioned in this setting41.
While at first view defining COx might seem simple and easy to perform at the bedside, some major barriers have to be faced.
The use of NIRS for quantifying cerebral autoregulation is based on some assumptions, and this continues to be a major point of discussion. ScO2 is determined by arterial blood oxygen content, cerebral oxygen consumption, oxygen-tissue diffusivity, and CBF. It has been assumed that, if all other parameters which influence ScO2 are kept constant, changes in ScO2 are due to changes in CBF and therefore that those two parameters can be interchanged. Although one might question this approach, the problem remains that to date there is no continuous real-time methodology to quantify CBF.
In most studies, COx is determined by using ICM+® software (Cambridge Enterprise Ltd, Cambridge, UK, http://www.neurosurg.cam.ac.uk/icmplus). The ScO2 signal and the MAP signal are captured longitudinally (time-domain analysis). Filtering of both signals is performed to limit analysis to the frequency of slow waves (0.05–0.003 Hz), which are relevant to autoregulation and exclude confounding wave components such as respiratory and pulse frequencies. A sliding analysis window of a 300-second period updated every 10 seconds runs across the signals, and the ScO2 and MAP data within these windows are plotted against each other, incorporating 30 data points for each calculation. The linear regression line for these data is computed, and the Pearson correlation coefficient is obtained. The process repeats as the analysis window scans over the signals in incremental steps, resulting in a continuous COx measurement.
To calculate the optimal MAP, the COx values are binned in 5 mmHg blood pressure increments. The first and last MAP bins, as well as bins which contain less than 2% of data points, are discarded. ICM+® then fits a U-shaped curve through the COx values plotted versus MAP. The optimal MAP can be identified at the point with the lowest COx.
However, owing to the granular nature imposed on the bin-aggregated data, binning makes it difficult to produce a robust automated algorithm to assess autoregulation42. The binned data are generally noisy in a clinical environment, which may be device-specific (for example, arterial line flushing or poor NIRS signal) or physiological in nature (for example, caused by administration of drugs or positional changes). This becomes even more important when very few bins contain data points, such as early in a procedure when the complete picture of the COx plot has not yet built up, or when blood pressure is relatively stable. Montgomery et al. demonstrated that the data in their raw format (that is, unbinned) are feasible for monitoring cerebral autoregulation, and they hypothesized that their method might be particularly useful in noisy environments42.
The current methodology for analysis of cerebral autoregulation includes either a time-domain or a frequency-based approach. The most common method is to calculate the correlation coefficient between ScO2 and MAP (time-domain approach). However, it could be argued that cerebral autoregulation is a complex physiological system and that simple correlation analysis does not cope with the complex interplay and the time-varying aspects of the different physiological mechanisms43. Coherence and transfer function analyses have also been used to quantify cerebral autoregulation44,45. Caicedo et al. analyzed four different measurement models used for cerebral autoregulation assessment (correlation, coherence, modified coherence, and transfer function). Although Caicedo et al. proposed transfer function gain as the most robust method when used for cerebral autoregulation studies, correlation was also considered a robust method, despite some restrictions related to time delay46.
Varying definitions to decide between intact and impaired autoregulation are found in the literature. Generally, a COx of less than 0.3 is considered consistent with intact autoregulation and the MAP with the lowest COx is considered the optimal MAP. However, noise on the data may impede the identification of intact and impaired regions. A further complication for the assessment of autoregulation is that, owing to interpatient variability, no specific threshold will apply to everyone. Moreover, it has been postulated that the flat part of the autoregulation curve is never really horizontal, but slightly tilted47. So autoregulation is not an on–off phenomenon, in which correlations between CBF and MAP jump from zero (intact autoregulation) to one (impaired autoregulation), and this makes the interpretation of autoregulation data rather cumbersome.
One approach to assess cerebral autoregulation is to monitor the ScO2 response to natural slow variations in blood pressure. However, the natural blood pressure variations may not be strong enough to challenge CBF. An alternative approach is to induce blood pressure variations (for example, by vasoactive drugs12, change in body position48, or release of a thigh cuff49) and measure the concomitant response of ScO2. However, it has been demonstrated that CBF dynamics are driven by the rate of change in blood pressure rather than absolute pressure per se; therefore, the precipitous change in pressure might elicit autoregulatory reactions that would not be seen with gradual changes in pressure12,50.
Despite the emerging availability of bedside physiology monitors, blood pressure targets remain mostly empirically chosen during critical care management. Efforts at preventing cerebral injury have emphasized the importance of maintaining a ‘normal blood pressure’ to ensure adequate perfusion of the brain. Hence, often medications are given to increase blood pressure in order to restore cerebral perfusion. However, as discussed before, the position and the shape of the autoregulation curve may have shifted away, with LLAs and ULAs which vary greatly and unpredictably14 and an autoregulation plateau which may be very narrow15. The clinical implication is that CBF might fluctuate as blood pressure fluctuates, exposing the patient to the risk of decreased cerebral flow and potential ischemia when blood pressure is relatively too low and conversely to the risk of increased cerebral flow and increased ICP when blood pressure is relatively too high. Since the individual limits of autoregulation are unknown, it is likely that empiric blood pressure management will result in patients having a MAP outside the autoregulatory range for at least part of the surgery or stay in intensive care. If autoregulation monitoring can individualize blood pressure targets, it could provide a more effective means for preventing ischemia–reperfusion injury than the current standard of care.
With the sophistication of signal processing and online analysis software, the challenge lies in the successful integration of ‘optimal MAP’ and autoregulation monitoring into daily critical care management.The next steps will be to study the autoregulation in different clinical situations and to clarify whether incorporation into therapeutic management protocols leads to an improved cerebral outcome.
Progress in perioperative and critical care will depend on moving away from broad assumptions and ‘one size fits all’ physiological targets. In the near future, real-time cerebral autoregulation monitoring may provide clinicians with the opportunity to individualize blood pressure targets and might direct critical care management to the individual patient’s need.
AM has received lecture fees from Medtronic (INVOS) and Sorin (NIRO). SD declares that he has no competing interests.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 31 Aug 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)