Keywords
androgenic, androgenetic, alopecia, hair loss, shedding, baldness
androgenic, androgenetic, alopecia, hair loss, shedding, baldness
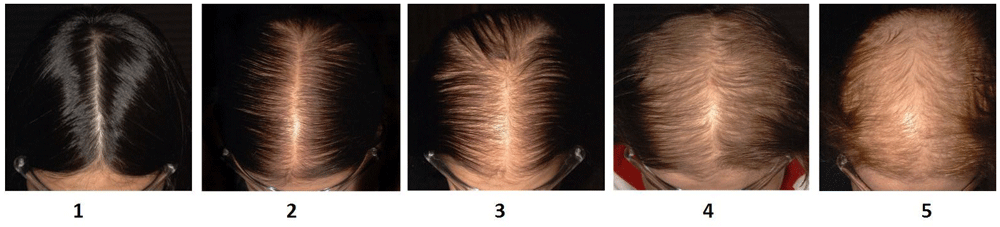
Hair shedding severity can be scored using a visual analogue scale1. For women with long hair, a shedding severity of 1, 2 or 3 is considered normal; severity 4 is borderline; while a shedding severity of 5 or 6 is excessive (Figure 1). Visual inspection of shed hair bulbs will determine if the hairs shed are being lost during the anagen phase of the hair cycle or the telogen phase. This examination of the bulb provides an important clue to the aetiology of the hair loss2.
Telogen effluvium is a non-scarring alopecia characterised by excessive shedding of telogen club hair diffusely from the scalp. It generally begins 8–12 weeks after a triggering event, such as pregnancy, major illness or complicated surgery, and resolves within 3–6 months. Once resolved, self-limiting telogen effluvium can be retrospectively diagnosed as acute telogen effluvium3. Telogen shedding that persists beyond 6 months is called chronic telogen effluvium (CTE)4. CTE may be primary or secondary to a range of triggers, including androgenetic alopecia (AGA), nutritional deficiency, endocrinopathy, connective tissue disease or drug induced5. The aetiology of primary CTE is unknown4. Mathematical modelling of CTE implicates a reduction in anagen duration variance in the pathogenesis6 and not due to an overall reduction in anagen duration, which is a feature of AGA7. The natural history is for continued hair shedding over many years. There may be some seasonal variation in the intensity of hair shedding8. Long-term follow up studies of women with primary CTE9 and histomorphometric and immunohistochemical examination of scalp biopsies in patients with both female pattern hair loss (FPHL) and CTE have confirmed that primary CTE is not a prodrome to AGA10. Nevertheless some women with longstanding CTE will develop con-incidental AGA as they age11.

Scores vary between 1 (minimal shedding) to 6 (copious hair shedding)12.
Primary CTE most commonly occurs suddenly in females between 30 and 50 years of age. Additional clinical features commonly seen in primary CTE include bi-temporal recession of the anterior hairline, a reduction in the thickness of their ponytail diameter4 and trichodynia13. Widening of the central part line suggests AGA, and is not a feature of primary CTE (Figure 2).
Other than identification and treatment of a triggering event, such as hypothyroidism, there is no known treatment for acute or chronic TE14. Treatments commonly used for AGA, such as finasteride, cyproterone acetate, spironolactone and flutamide, do not work in TE5.
Topical minoxidil has been used for over 30 years to treat a variety of hair loss conditions, including AGA. For some years in our clinic we have also used minoxidil orally to treat men and women with androgenetic hair loss, who are either intolerant to topical minoxidil or do not like the look or feel or minoxidil in their hair15. As Minoxidil is only available in Australia as a 10 mg tablet (Loniten), we compounded minoxidil extemporaneously in various doses ranging from 0.25mg to 2.5mg. More recently, a case report demonstrated that low dose oral minoxidil was useful in treating a female with chemotherapy-induced alopecia with oral minoxidil16. There have been no reports or studies that examine the use of oral minoxidil in CTE. Our understanding of the pathogenesis of CTE and the mechanism of action of minoxidil on hair growth suggest it should work in CTE patients, and we have found that women with AGA who are unresponsive to topical minoxidil often respond to oral minoxidil at our clinic. This retrospective study examines the use of low-dose oral minoxidil in women diagnosed with CTE.
Examination of patient records for this retrospective chart review was conducted at Sinclair Dermatology practice in Melbourne, Australia. As this was a retrospective review of patient charts, it did not require prior approval from our local institutional ethics committee. The data are the property of Sinclair Dermatology and access was approved by the company. Records between Jan 2012 and Oct 2015 were extracted. Inclusion criteria included female patients with a hair shedding score (HSS)12 of 4–6 without visible mid frontal scalp hair loss (Sinclair stage 1)17 and no hair follicle miniaturization on scalp biopsy. Patients were excluded if they were using other treatments for hair loss whilst taking oral minoxidil. Data pertaining to dosage of oral minoxidil, previous treatments, including topical minoxidil use, blood pressure, side effects, hypertrichosis and trichodynia were extracted from the records. Patient subjective responses were based on a HSS score (Figure 1) and were recorded at each consultation. The HSS scores prior to starting oral minoxidil, and scores at 6 and 12 months were extracted for analysis.
Data were analysed using Matlab R2014b statistical software. Difference in HSS at baseline, 6 and 12 months were analysed using the Wilcoxon rank sum test for pair-wise comparisons. Differences in blood pressure at baseline and 6 months were also analysed using Wilcoxon rank sum test. Relationship between outcomes (HSS score at 6 and 12 months) and individual patient specific variables, including age, topical minoxidil, duration of disease, dosage of minoxidil and HSS score at baseline were analysed using a generalised linear regression model.
Thirty-six patients with CTE, who were prescribed oral minoxidil, were included in this analysis. The mean age was 46.9 years (range 20–83 years) and the dosage of oral minoxidil used varied between 0.25 and 2.5 mg with most patients being administered 1 mg. Mean baseline HSS was 5.64. Mean HSS scores improved at 6 and 12 months at 3.9 and 3.05, respectively (Figure 3). There was a reduction in mean HSS scores from baseline to 6 months of 1.7 (p<0.001) and a reduction in mean HSS scores from baseline to 12 months of 2.58 (p<0.001). Similarly, a mean reduction of 0.89 in HSS scores was noted between 6 months and 12 months (p =0.003). Correlation between the duration of disease and previous topical minoxidil with HSS scores at 6 months (R2 < 0.22) and 12 months (R2 < 0.11) were weak. Eleven patients had previously used 5% topical minoxidil. Of these patients the mean change in the HSS score was higher, although not statistically significant compared to patients who did not use topical minoxidil: mean reductions in HSS score for patients who had previously used and not used topical minoxidil were 2 and 1.56 (p= 0.22) at six months and 3.18 and 2.32 at 12 months (p=0.11). The HSS score improved in 31 patients after 6 months; in 4 patients the HSS score remained the same; and in 1 patient the score increased at the 6-month mark before improving, compared to baseline, at the 12-month mark. After 12 months the HSS score remained equal or improved from baseline in all but 3 patients.
There were no significant differences between blood pressure at baseline and 6 months (p>0.05). The lowest blood pressure recorded was 90/70. All other patients in the study had a blood pressure above 100/70. Mean change in blood pressure was minus 0.5 mmHg systolic and plus 2.1 mmHg diastolic. Two patients developed transient postural dizziness that resolved with continued treatment. One patient developed ankle oedema. Fourteen women developed facial hypertrichosis. For 6 women this was mild and did not require treatment; 4 patients waxed their upper lip or forehead; and 3 patients had laser hair removal. No patients developed any blood test abnormality. Five women described trichodynia at baseline and all noted improvement or resolution within 3 months.
CTE is the persistent shedding of telogen hairs diffusely from the scalp, which is associated with bitemporal recession without widening of the central part. CTE was described as a primary idiopathic disease entity by Whiting in 19964. The prognosis for women with CTE is uncertain and women do not go bald8. Rather, the disease has a fluctuating course and the condition may continue for many years. The goal of this retrospective study were to review the use of oral minoxidil in CTE with respect to the response in HSS score and safety.
Minoxidil is a piperidinopyrimidine derivative and a potent arteriolar vasodilator5. Studies examining oral minoxidil demonstrated more rapid and extensive hair growth, compared to topical treatment, in patients with alopecia areata12. A recent study examined the use of oral minoxidil in combination with spironolactone in 100 women with FPHL15. The study demonstrated a reduction of hair loss and hair shedding at 6 and 12 months. Dosages of 0.25 mg of oral minoxidil were used in that study.
Use of oral minoxidil for hair loss has not been extensively reported in the literature to date. Currently, the only suggested treatment for CTE is topical Minoxidil, however results are variable and often disappointing. One study demonstrated an improvement in 55.2% of patients studied, using 5% topical minoxidil for men and 5% topical minoxidil with 50 mg of cyproterone acetate for women14. 25.2% of patients had a moderate response to treatment14.
All the patients in this study demonstrated an improvement at either the 6 month or 12 month mark, with 33 patients improved from baseline at the 12 month mark.
FPHL: female pattern hair loss, AGA: androgenetic alopecia, CTE: chronic telogen effluvium, HSS: Hair shedding score.
Dataset 1: Raw data collected during the study. DOI, 10.5256/f1000research.11775.d17641818.
Rodney Sinclair is director of Samson Clinical Pty Ltd, owner of PCT Patent Application PCT/AU2015/050682, Entitled: Detection and treatment of excessive hair shedding. The invention relates to a method of treating telogen effluvium in a subject by administering to a subject an oral dose of minoxidil.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 06 Sep 17 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)