Keywords
malaria, Plasmodium, falciparum, multiplicity of infection, allele, msp2, Sudan
This article is included in the Pathogens gateway.
malaria, Plasmodium, falciparum, multiplicity of infection, allele, msp2, Sudan
Malaria was and still is a major health problem, with over one third of worldwide populations being at risk (World Health Organization, 2015). The burden of this disease falls the heaviest on Sub-Saharan African countries (World Health Organization, 2015). In Sudan, almost 90% of the population live under high malaria transmission levels, as recently reported by the WHO (“WHO | Sudan,” 2016; World Health Organization, 2015). Malaria in Sudan is transmitted mainly by Anopheles arabiensis and the majority of cases are due to P. falciparum (Ageep et al., 2009). The merozoite surface protein-2 (MSP2) is a highly polymorphic plasmodium membrane protein and the most expressed in the surface of the merozoite (Gerold et al., 1996). It has been long studied in vaccine trials for being accessible by the immune system (McCarthy et al., 2011). MSP2 protein is highly polymorphic, which gives an opportunity for molecular epidemiologists to differentiate between recurrent and new infections (Mwingira et al., 2011). Genetic diversity of MSP2 alleles is due to mutations and proliferation of highly polymorphic central repeats (Ferreira & Hartl, 2007). The allelic diversity of MSP2 proteins can be used to calculate multiplicity of infection (MOI), which is the number of different strains of P. falciparum co-infecting the same host and is a valuable for estimating malaria transmission level (Vafa et al., 2008). Sudan experiences a range of climate variations, from arid desert in the north, to tropical areas in the south and a short rainy season (Noor et al., 2012). The current study aimed to determine the level of genetic diversity of the P. falciparum MSP2 gene, using field isolates across different geo-ecological regions in Sudan. The study also aimed to determine the relationship between genetic diversity and the characteristics between different patients. Little has been documented regarding the molecular diversity of P. falciparum in hypo and meso-endemic regions of Sudan, in particular after adoption of ACT as antimalarial treatment.
This study was conducted across the two geographical zones of Sudan: the semi-desert regions of the Red Sea (Northeast) and the savannah regions of Khartoum (Central), Gezira and River Nile states. It took place between August 2011 and December 2013 – peak malaria transmission is between July and December (rainy season) in the poor and rich savannah regions, but in the semi desert regions of the Red Sea, peak malaria transmission occurs between November and January, during the winter season.
Ethical clearance was obtained from the scientific and research ethics committee, Institute of Endemic Diseases, University of Khartoum. Informed consent was obtained from patients, or guardians if the patient was a minor, for participation in this study. Anonymity and confidentiality of patient information were maintained throughout.
A total of 271 symptomatic malaria patients from all age groups regardless of gender were recruited from selected health facilities (Omdurman teaching hospital and Omdoom and Mubarak Zaroog health centers in Khartoum, Wad madani teaching hospital in Gezira, Zeedab, Kaboushia and Elbouga hospitals in River Nile and Unity hospital in Red Sea) during peak malaria transmission season. Patients were passively recruited from the outpatient clinics of these hospitals during the study period. Patients were diagnosed with malaria using blood film microscopy. Parasite density and hemoglobin level were measured on each sample upon collection. Thick and thin blood smears were stained with Giemsa stain. Parasite density was determined by counting the number of asexual parasites per 200 white blood cells using the formula described by Cook (Cook, 1990). The data was grouped based on the level of parasitemia, into low = 1–5,000 parasites/µl, intermediate = 5,001–10,000 parasites/µl and high = >10,000 parasites/µl.
Parasite DNA was extracted from dried blood samples spotted on filter papers using a method described by Musapa et al. (Musapa et al., 2013). Plasmodium species were identified by 18S rDNA based nested PCR, using genus and species specific primers manufactured by Macrogen, Korea, as described by Snounou et al. [15]. Msp2 alleles were further amplified with slight modification of the standardized nested-PCR protocols described previously by Snounou et al. (Snounou et al., 1993)
The amplified PCR products were run on 2% Agarose gel (Caisson, Utah, USA) stained with 4µl ethidium bromide at 100V and 30A for 60 minutes. DNA fragments were estimated using 100 bp DNA ladder marker (Vivantis, Selangor DarulEhsan, Malaysia) and the bands were viewed under UV light using trans-illuminator (BioDoc-It UVP, Cambridge, UK).
The multiplicity of infection (MOI) was calculated by dividing the total number of fragments observed in MSP2 by the number of positive samples. Isolates with a single genotype were considered monoclonal infections while isolates with more than one genotype was considered multiclonal infections.
Data was analyzed using SPSS version 20 (SPSS, Inc., Chicago, IL, USA). Proportions were compared for significance using the χ2-test. The association between MOIs, parasite densities and age groups were computed using Spearman’s rank correlation coefficient. Statistical significance among MOIs and mean hemoglobin levels for the different age groups were calculated using the Kruskal-Wallis H-test at a P-value of ≤ 0.05.
P. falciparum rDNA was detected in a total of 241 (88.9%) malaria patients using nested PCR (Figure 1). 158 (65.6%) were male, while 83 (34.4%) were female. The distribution of patients across the four studied states: Khartoum, Gezira, River Nile and the Red Sea was 81 (33.6%), 58 (24.1%), 47 (19.5%) and 55 (22.8%), respectively. The age groups ranged between 3–90 years, with a mean of 31.39 ±14.9 years. The mean parasite density was higher in the River Nile state (15869 parasites/µl) compared to Khartoum, Gezira, and Red Sea states (14563, 11266, and 14603 parasites/µl, respectively), and this difference was statistically significant (p-value = 0.02). The mean hemoglobin level was lowest in Khartoum (5.23 g/dl) compared to the other regions; Gezira, River Nile, and Red Sea (7.94, 9.26, and 7.74 g/dl, respectively), and the difference was statistically significant (p-value = 0.02) (Table 1).
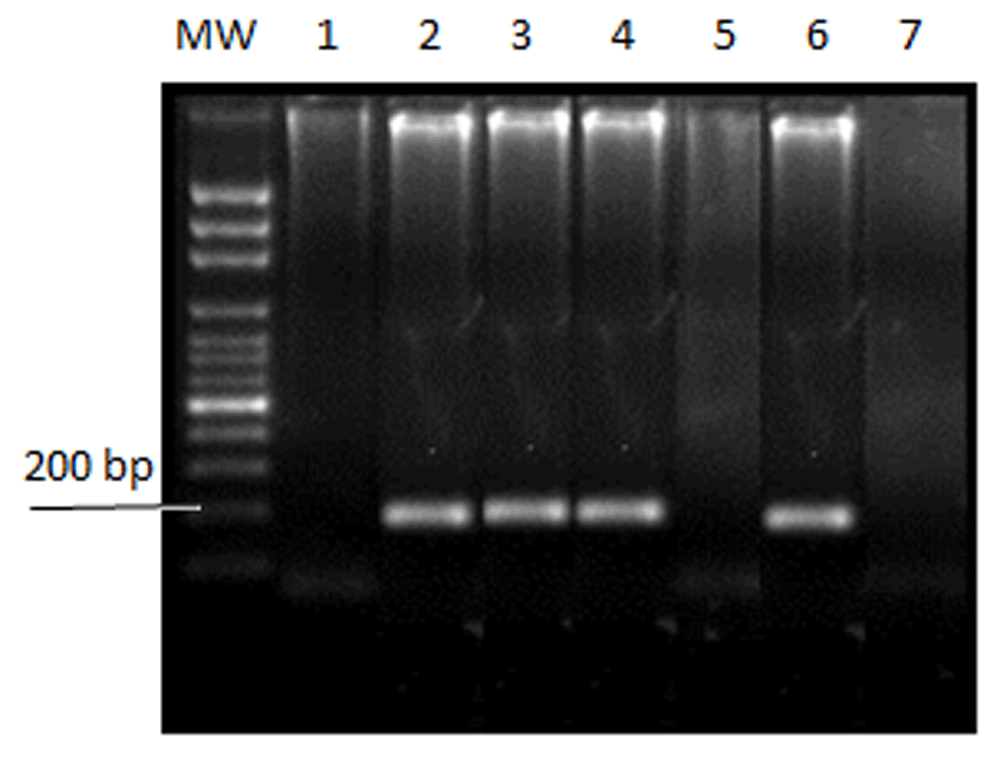
Amplification of the P. falciparum ribosomal DNA gene using genus and species specific primers by nested PCR, yielding amplicons of 220 bp in size.) Molecular weight marker (MW) is 100 bp (iNTRON, Inc.). Lanes 2, 3, 4, and 6 are positive for samples of P. falciparum; 1 and 5 are negative for samples of P. falciparum; lane 7 is the negative control.
Allele genotyping revealed the highly polymorphic nature of Sudanese P. falciparum isolates with respect to the MSP2 gene. Both IC1/3D7 and FC27 MSP2 allele types were identified (Figure 2). The total number of different sized alleles detected in this study was 42. Among them IC1/3D7 (160–400 bp) and FC27 (140–400 bp) allele families were noted. Frequencies of different MSP2 alleles and their combinations and multiplicity of infection across the study sites are shown in Table 2. The frequency of samples with only IC1/3D7 and FC27 were 56.4% (136/241), and 17% (41/241), respectively and IC1/3D7/FC27 combinations were found in 26.6% (64/241) of samples. The prevalence of IC1/3D7 and FC27 allelic types was 82.9% (200/241) and 43.5% (105/241), respectively. Multiple clones were detected in all study sites. Multiplicity of infection (MOI) was highest in P. falciparum infected patients from Gezira state (1.67 genotypes per infection) and lowest in those from Red Sea state (1.20 genotypes per infection) and it was statistically significant, (p-value = 0.001) (Table 2). The difference in distribution of allelic polymorphism of MSP2 was only significant for total MSP2 and FC27 (p-value = 0.002 and p-value = 0.042, respectively). However, for IC1/3D7 the result was not statistically significant (p-value = 0.89). The estimated mean MOI of all studied areas was 1.46 genotypes per infection (Table 2). There was a negative correlation between age and parasite density (Spearman rank coefficient = 0.03; p-value = 0.65), though it was not significant. The 18–40 age group had the highest mean parasite density (16354 parasites/µl, Table 3).
| allelic type | Khartoum | Gezira | River Nile | Red Sea | Total | Fragment size* | No. of alleles | p-value |
|---|---|---|---|---|---|---|---|---|
| n=81 | n=58 | n=47 | n=55 | n=241 | ||||
| n(%) | n(%) | n(%) | n(%) | n(%) | ||||
| IC1/3D7 | 42(51.9) | 21(36.2) | 28(59.6) | 45(81.8) | 136(56.4) | 450–750 | 11 | |
| FC27 | 14(17.2) | 14(24.1) | 10(21.3) | 3(5.5) | 41(17.0) | 250–500 | 10 | |
| IC1/3D7/FC27 | 25(30.9) | 23(39.7) | 9(19.1) | 7(12.7) | 64(26.6) | |||
| 81(100) | 58(100) | 47(100) | 55(100) | 241(100) | 21 | 0.002 | ||
| Total IC1/3D7 | 67(82.7) | 44(75.9) | 37(78.2) | 52(94.5) | 200(82.9) | 0.893 | ||
| Total FC27 | 39(48.1) | 37(63.8) | 19(40.4) | 10(18.2) | 105(43.5) | 0.042 | ||
| Multiclonal isolates | 25(30.9) | 23(39.7) | 9(19.1) | 7(12.7) | 64(26.6) | |||
| MOI | 1.58 | 1.67 | 1.32 | 1.20 | 1.46 | 0.001 |
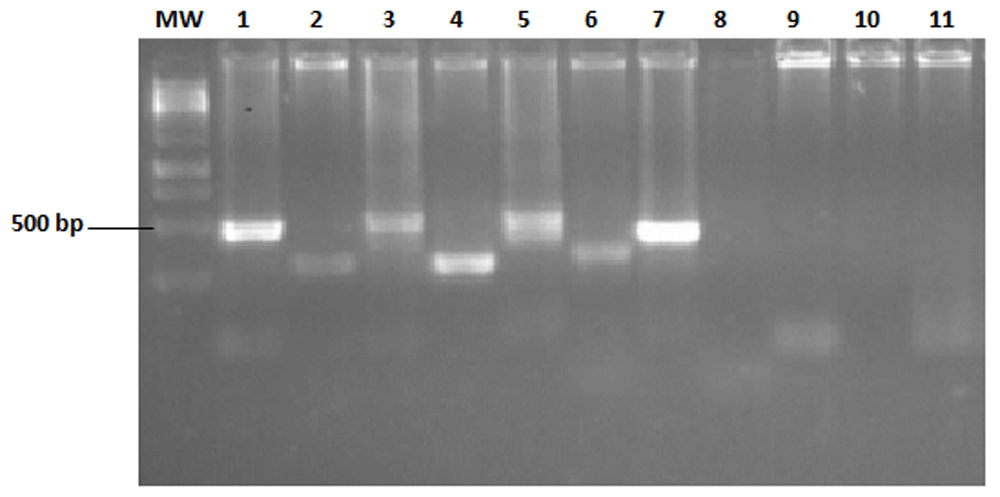
PCR typing of MSP2 multiple clones (FC27) band size range (250–400 bp) and (IC1/3D7) band size range (500–750 bp). MW: 100 bp ladder (iNTRON, Biotechology). Lanes 1, 3 and 7 are positive for IC1/3D7, lane 9 is negative for the IC1/3D7 allelic family. Lanes 2, 4, and 6 are positive for the FC27 allelic family, 8 is negative for FC27 and lane 11 is a negative control. Lane 5 is positive for IC1/3D7, showing multiple clones (500 and 600 bp).
The distributions of P. falciparum MSP2 block 3 allelic types across different age groups are shown in Table 3. The prevalence of IC1/3D7 and FC27 is shown in the same table.
There is no significant correlation between multiplicity of infection and the age group the patient is in (Spearman rank coefficient = 0.01; P-value= 0.50). The distribution of IC1/3D7 and FC27 according to different levels of parasitaemia is shown in Table 4. Neither the individual distribution of IC1/3D7and FC27 nor the multiclonal isolates were significantly different among different parasitaemic groups (p-value = 0.7 and p-value = 0.957, respectively).
Increasing our knowledge of the genetic diversity of P. falciparum induced malaria will certainly help us understand its pathogenesis, acquired immunity and drug resistance. 14, 15, 13 and 12 different alleles of MSP2 were identified in Khartoum, Gezira, River Nile and Red Sea states, respectively. This data is consistent with previous studies in low and unstable malaria transmission regions in eastern Sudan (A-Elbasit et al., 2007), central Sudan (Hamid et al., 2013), and other areas of seasonal unstable malaria transmission (Elmahdi et al., 2012). 3D7 alleles were more prevalent in the four states (Khartoum, Gezira, River Nile and Red Sea) compared to FC27 alleles. This finding differs from those of previous studies in central and eastern Sudan, where FC27 was the most predominant allelic family (A-Elbasit et al., 2007; Babiker et al.,1997; Hamid et al., 2013). In our study, 25% of studied samples were found to have mixed infections with multiple parasite clones; a result similarly reported in eastern Sudan (Babiker, 1998).
In our study, multiplicity of infection was highest in the >40 age group (with an average MOI of 2 and 1.68 in Khartoum and Gezira states, respectively), whereas the MOI was highest in the <18 age group (with an average of 1.37 and 1.33 in River Nile and Red Sea states, respectively), although the difference was not statistically significant. Recent studies on the variation of MOI with age have suggested that the influence of age on MOI is highly affected by the endemicity of malaria (Pinkevych et al., 2015). this is consistent with studies that have shown an age-dependent MOI in villages with intense perennial malaria transmission (Smith et al., 1999) and some areas with hypo-meso-endemic malaria transmission like Ghana (Agyeman-Budu et al., 2013),
The relatively higher number of alleles detected at high parasite densities might also mean that more diverse parasite populations are present in such infections. This suggestion is partly supported by the finding that all the different allelic types were more often concurrently detected in the high-density samples (Färnert et al., 2001). The present study reported that the increasing level of multiplicity was seen with increased parasite density of the samples. These findings are compatible with a previous study that has shown a clear trend of increasing parasite density with increased multiplicity for genetic markers on both genes, MSP1 and MSP2 (Peyerl-Hoffmann et al., 2001). The results of our study will likely influence current and future malaria control strategies since MOI can predict antimalarial treatment response (Kyabayinze et al., 2008).
Allele genotyping revealed the highly polymorphic nature of Sudanese P. falciparum isolates with respect to the MSP2 gene. The results of our study are expected to have an influence on the current and future malaria control strategies, since MOI predicts development of clinical malaria and subsequent efficacy of antimalarial treatment.
MSP2: merozoite surface protein 2, PCR: polymerase chain reaction, MOI: multiplicity of infection, ANOVA: analysis of variance, WHO: World Health Organization, rDNA: ribosomal DNA, ACT: artemisinin based combination therapy.
The study received approval from the scientific and research ethics committee of the Institute of Endemic Diseases; University of Khartoum, Sudan. Informed consent was obtained from all participants (or from the parents/legal guardians when the participant was a minor), prior to their enrolment. All malaria patients approached in the study were treated using the standard treatment of WHO protocol for malaria (Olumese, 2017).
Dataset 1: Raw data supporting the findings presented in this study. The dataset is available both in XLSX and SPPS format. DOI, 10.5256/f1000research.12585.d179181 (Mustafa et al., 2017).
This study was partially funded by the National Malaria Program Administration, Ministry of Health, the University of River Valley, Sudan and Third World Academy of Science (TWAS), Trieste, Italy [project no. 13-145 RG/BIO/AF/AC_G].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank all malaria patients who participated in the study. The authors would also like to thank Prof. Muntaser E. Ibrahim, director of the Dr. Douglas Barker Molecular Biology Lab, Institute of Endemic Diseases, University of Khartoum, for hosting the molecular work and data analysis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Mustafa S, Abdel Hamid M, Aboud M, Amin M, et al.: Genetic diversity and multiplicity of Plasmodium falciparum merozoite surface protein 2 in field isolates from Sudan. F1000Research. 2017; 6. Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Plasmodium falciparum drug resistance
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 02 Oct 17 |
read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)