Keywords
endotoxin, acute lung injury, interstitial pneumonia, heart failure
endotoxin, acute lung injury, interstitial pneumonia, heart failure
Bovine pulmonary hypertension is associated with arterial hypoxemia, systemic arterial hypotension, and increased central venous pressure1,2. These physiological changes are conducive to tissue ischemia. This may be particularly deleterious to tissues, such as the intestinal mucosa, that must function in low oxygen environments under normal physiological conditions3. Impaired intestinal mucosal barrier function may, therefore, be a sequela of rising mean pulmonary arterial pressures in cattle progressing through the confined feeding period of production. If so, mucosal ischemia may promote the translocation of bacteria and toxins across the intestinal wall and into the mesenteric lymph or portal venous circulations. Reduced mucosal barrier function may be a component cause for numerous diseases of feedlot cattle approaching slaughter weight such as liver abscess formation. The goal of this study was, therefore, to evaluate the effect of hypoxia-induced pulmonary hypertension on intestinal mucosal barrier function in a Holstein calf model. We hypothesized that hypoxia-induced pulmonary hypertension would be associated with increased intestinal permeability.
Six, 2-month old male, intact, clinically healthy Holstein dairy calves were collected from a farm in West Texas. Calves were housed for 2-weeks under normoxic (975 m altitude) or hypoxic (4,570 m altitude) conditions. Pulmonary arterial pressures were measured on Days 0 and 14 of the study. Calves were euthanized on Day 15, and lung tissue was collected for histology. Pulmonary arteriolar remodeling was semi-quantitatively scored. The study was approved by the Texas Tech University Institutional Animal Care and Use Committee (Protocol 16109-12). All efforts were made to ameliorate any suffering experienced by the animals in this study through daily observations and recording of animal health.
Male, intact Holstein calves were obtained from one commercial dairy farm in West Texas (n = 6). Calves were born and raised on the dairy farm until collection at 2 months of age. The calves were clinically healthy, but the farm calf manager reported a recent outbreak of bloody scours among other calves on the farm. Calves were fed 4 to 5 liters of colostrum within 24 hours of birth and provided with 2.8 L of milk twice per day until weaning at 60 days of age. From 2 weeks of age they were provided with ad libitum access to a pelleted complete ration calf starter (≥ 20% crude protein, dry matter basis).
Calves were weighed on arrival at the Texas Tech University farm (altitude: 975 m) and randomly allocated to one of two pens stratified by body mass (Agri-Plastics, Grassie, ON, Canada). The hypoxic group (n = 3) was housed on a raised slatted floor inside a temperature-controlled chamber (temperature 17 ± 3 °C) (dimensions: 1.8 m × 2.3 m). The normoxic control group (n = 3) was housed in a shaded outdoor pen (1.8 m × 3.5 m) with straw bedding on a sloped concrete floor. The pen was moved to a new location every 3 days, and the inside of the pen cleaned was cleaned with a virucidal disinfectant (Virkon S, DuPont, Wilmington, DE). Soiled straw was removed daily and all straw was replaced every 3 days. Maximum and minimum daily temperatures experienced by the control calves during the study ranged from 11 to 30°C and from 5 to 15°C, respectively. After a 5-day acclimation period, the air within the chamber housing the hypoxic group was reduced to 14% oxygen, simulating an altitude of 4,570 m (Day 1 of the study). Calves were provided ad libitum access to water and a pelleted complete ration calf starter (≥ 20% crude protein, dry matter basis).
Two control calves (calves 2 and 3) started shedding occult fecal blood on Day 3; consequently, all calves were treated for coccidiosis over a 5-day period starting on Day 5. Calves were given amprolium in drinking water at a rate of 47 mL per 10 gallons of water, providing approximately 10 mg amprolium per kg body mass at the usual rate of water consumption. Fecal shedding of blood ceased on Day 8 and feces returned to normal color and consistency on Day 9. Calves did not show signs of tenesmus and maintained a normal appetite.
Pulmonary arterial pressure testing was performed on Days 0 and 14. The neck was cleaned with chlorhexidine solution before a 12 gauge, 8.9 cm hypodermic needle was inserted into the jugular vein. Flexible, saline-filled polyethylene catheter tubing (external and internal diameter of 17 and 12 mm, respectively) was then fed through the needle and into the jugular vein. A pressure transducer (TranStar DPT, Smiths Medical ASD, Inc., Dublin, OH) connected the catheter and oscilloscope (BM5Vet, Bionet America, Inc. Tustin, CA, U.S.A.). The change in the pressure waveform that occurred as the catheter tip was advanced through the right atrium, right ventricle, and finally into the pulmonary artery was monitored on the oscilloscope. The jugular vein, right atrium, right ventricle and pulmonary artery have distinct pressure waveforms.
Arterial blood-gas analyses were performed to verify the hypoxic status of the calves exposed to hypoxic and normoxic conditions. Samples were collected from all calves on Days 0 and 14. Approximately 1 to 3 mL of blood was collected from the auricular artery using a 20 gauge, 2.5 cm hypodermic needle attached to a pre-heparinized 3 mL syringe. Air bubbles were immediately expelled and the first several drops of blood discarded before analysis on a portable analyser (VetScan i-STAT 1, Abaxis, Union City, CA, USA). Blood-gas tensions were adjusted according to rectal temperature.
Intestinal permeability to the synthetic substances D-Mannitol (100%) (Fisher Scientific, Bridgewater, NJ) and lactulose (99%, Alfa Aesar, Ward Hill, MA) were evaluated twice: on Days 0 and 14. Poly-vinyl tube (outer diameter 0.64cm) was used as a nasogastric tube to administer the substances dissolved in 60 mL of warm water (15 g lactulose and 5 g mannitol). The calf’s head was restrained to one side before the nasogastric tube was inserted into the nostril and advanced caudo-ventrally so that it passed along the ventral meatus, through the nasopharyngeal opening and into the esophagus. The lactulose and mannitol was syringed into the tube. Approximately, 5 mL of air was used to clear all remaining fluid from the nasogastric tube before it was removed.
A 16 gauge, 5 cm catheter was placed in the jugular vein to facilitate the collected of blood at 0, 2, 4, 6, and 8 hours following the administration of the lactulose and mannitol. Blood was collected in red stopper blood tubes (10 mL) and the serum stored (-20 °C) within approximately 1 hour of collection.
To extract serum proteins in preparation for Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) analysis, samples were thawed at room temperature and 100 µL of homogenized serum sample transferred to a microcentrifuge tube (2 mL, Eppendorf, Hauppage, NY, USA). Next, 300 µL of solvent mixture containing acetonitrile (LC/MS Optima Grade, Fisher Scientific, Bridgewater, NJ, USA) and water (LC/MS Optima Grade, Fisher Scientific, Bridgewater, NJ, USA) (80:20; v/v) was transferred to the tube. After 5 minutes at room temperature, the tube, now containing serum and solvent solution, was vortexed for 30 seconds prior to centrifugation at 21,130 × g for 10 min (5424 R, Eppendorf, Hauppage, NY, USA). The supernatant was transferred into a syringeless filter vial (PTFE, 0.45 µm, GE Healthcare UK Ltd., UK) for LC-MS/MS analysis. Standards were prepared using 10 mg of the standard in 10 mL (mannitol) or 15 mL (lactulose) of solvent (50:50; v/v; methanol:water). From these, serial dilutions of 1, 50, 10, 25, 50, 100 µg/mL in acetonitrile/water mixture (80/20; v/v) were prepared.
Ultra-High Pressure Liquid Chromatography-Mass Spectrometry (UPLC-MS) was performed on a liquid chromatograph with triple staged quadrupole mass spectrometer (TSQ-MS) (Ultimate 3000, TSQ Endura, Thermo Fisher Scientific, Waltham, MA). Serum extract (5 µL) was injected into an RP-Amide column (Accentis RP-Amide; 5 µm; 50 × 2.1 mm, Sigma-Aldrich, St. Louis, MO, USA). The column and autosampler tray temperatures were 45°C and 10°C, respectively. Mobile phases A and B consisted of 0.1% formic acid (LC/MS Optima Grade, Fisher Scientific, Bridgewater, NJ, USA) in water and 0.1% formic acid in acetonitrile, respectively. The flow rate was 0.4 mL/min. Mobile phase gradient information, mass spectrometry parameters, and transitions monitored for lactulose and mannitol analysis are provided (Table 1 to Table 3).
| Minute | Mobile phase A (%) | Mobile phase B (%) |
|---|---|---|
| 0 | 25 | 75 |
| 3 | 25 | 75 |
| 5 | 60 | 40 |
| 6 | 60 | 40 |
| 8 | 25 | 75 |
| 10 | 25 | 75 |
Calves were euthanized with intravenous pentobarbital sodium (85 mg/kg) on Day 15 of the study. The atria were separated from the ventricles at the atrioventricular junction. The right ventricular free wall (RV) was separated from the left ventricle and septum (LVS). The RV and LVS were individually weighed.
The right diaphragmatic lung lobe was perfused with formalin (10%, neutral buffered) at 15 to 20 cm H2O for approximately 5 minutes. After 5 days of formalin fixation, lung sections were collected midway along the dorsal aspect of the lobe for histology. Tissue from the caudate liver lobe was also preserved in formalin (10%). Tissue sections (4 µm) were stained with hematoxylin and eosin. Pulmonary arterioles (< 500 μm) were semi-quantitatively scored for medial hypertrophy and adventitial fibrosis (0 = no lesion; + 1 = mild; + 2 = moderate; + 3 = severe). The liver was evaluated for congestion, hydropic degeneration, lipidosis, and other miscellaneous lesions.
Statistical analyses were performed using a commercially available software (Stata version 12.1, College Station, TX). Summary statistics are presented as mean ± SE unless otherwise specified. Between group differences were evaluated using Student’s t-test, with equal variances. Student’s t-test is a suitable statistical method for small sample sizes (n ≤ 5) even if group sizes are unequal, as long as the effect size is expected to be large4. Generalized estimating equations with an exchangeable correlation structure were used to evaluate the effect of hypoxia (hypoxic versus normoxic) on serum levels of lactulose, mannitol, and the lactulose to mannitol ratio. Two-way interactions were evaluated between group and test and between group and time from lactulose and mannitol administration.
On Day 0, calf body masses ranged from 75.0 to 86.4 kg with a median of 76.4 kg. Mean body masses for hypoxic and control calves were 78.9 ± 2.8 kg and 79.2 ± 3.6 kg, respectively (P = 0.95). On Day 14, calf body masses ranged from 82.7 to 94.0 kg with a median of 90.4 kg. By the end of the study, the body mass of all calves had increased (P = 0.01), but the controls (93.1 ± 0.5 kg) were significantly heavier than the hypoxic calves (85.9 ± 1.7 kg) (P = 0.02).
Controls had greater mean right atrial pressures than hypoxic calves at Day 14 (P = 0.001) but not at Day 0 (P = 0.53) (Table 4). There was no difference in mean right ventricular pressures at Day 0 (P = 0.81) or Day 14 (P = 0.30). Control calves tended to have greater mean pulmonary arterial pressures than hypoxic calves on Day 0 (P = 0.17) but there was no difference between groups on Day 14 (P = 0.47). On average, mean pulmonary arterial pressure increased by 16 mm Hg (± 2 mm Hg) from Day 0 to 14 (P < 0.001).
Arterial pCO2 did not differ between control and hypoxic calves on Day 0 (P = 0.99) or Day 14 (P = 0.71) of the study (Table 5). Arterial pO2 did not differ between control and hypoxic calves on Day 0 (P = 0.99), but pO2 was significantly lower in hypoxic calves than controls on Day 14 (P = 0.005).
| PaCO2 (mm Hg) | PaO2 (mm Hg) | ||||
|---|---|---|---|---|---|
| Group | Calf | Day 0 | Day 14 | Day 0 | Day 14 |
| Control | 1 | 35 | 43 | 75 | 81 |
| 2 | 32 | 42 | 46 | 71 | |
| 3 | 39 | 27 | 51 | 81 | |
| Hypoxic | 4 | 33 | 49 | 50 | 58 |
| 5 | 34 | 31 | 49 | 52 | |
| 6 | 40 | 41 | 47 | 48 | |
The ratio of right ventricular mass to total ventricular mass was greater in hypoxic calves than controls indicating greater work hypertrophy (P = 0.04). Both hypoxic and control calves showed histologic lesions of mild (1+) to moderate (2+) medial hypertrophy of the pulmonary arterioles and zero to moderate adventitial fibrosis (Table 6). Two control calves (calves 2 and 3) had gross and histologic lesions consistent with interstitial pneumonia: heavy, wet lungs that failed to collapse (Figure 1) and diffuse alveolar damage (Figure 2). One hypoxic calf (calf 4) had extensive (3+) bronchial associated lymphoid tissue (BALT) and two control calves (calves 1 and 3) had moderate (2+) BALT proliferation. In all cases the BALT was only found around large bronchioles.
| Pulmonary arteriole | |||||
|---|---|---|---|---|---|
| Group | Calf | RV:TV* | Medial hypertrophy | Adventitial fibrosis | Liver |
| Control | 1 | 0.28 | 2+ | 1+ | Hepatic lipidosis (2+), congestion (zone 2) |
| 2 | 0.27 | 1+ | 0 | Congestion and sinusoid dilation (zones 1 and 2), hepatic lipidosis (1+) | |
| 3 | 0.31 | 1+ | 1+ | Congestion and sinusoid dilation (zones 1 and 2), hydropic degeneration (3+), multi-foci abscessation (2+) | |
| Hypoxic | 4 | 0.33 | 1+ | 1+ | Congestion and sinusoid dilation (zones 1 and 2) |
| 5 | 0.32 | 2+ | 2+ | Congestion and sinusoid dilation (zones 1 and 2), hydropic degeneration (1+) | |
| 6 | 0.32 | 1+ | 0 | Congestion and sinusoid dilation (zones 1 and 2), hydropic degeneration (1+), portal vein dilation (2+) | |

Gross lesions observed in a control calf (calf 2) that developed bloody scours 13 days prior to postmortem examination showing (A) reddened, inflamed intestines next to healthy intestine, (B) wet lungs that failed to collapse, and (C) heart showing a dilated right ventricle.
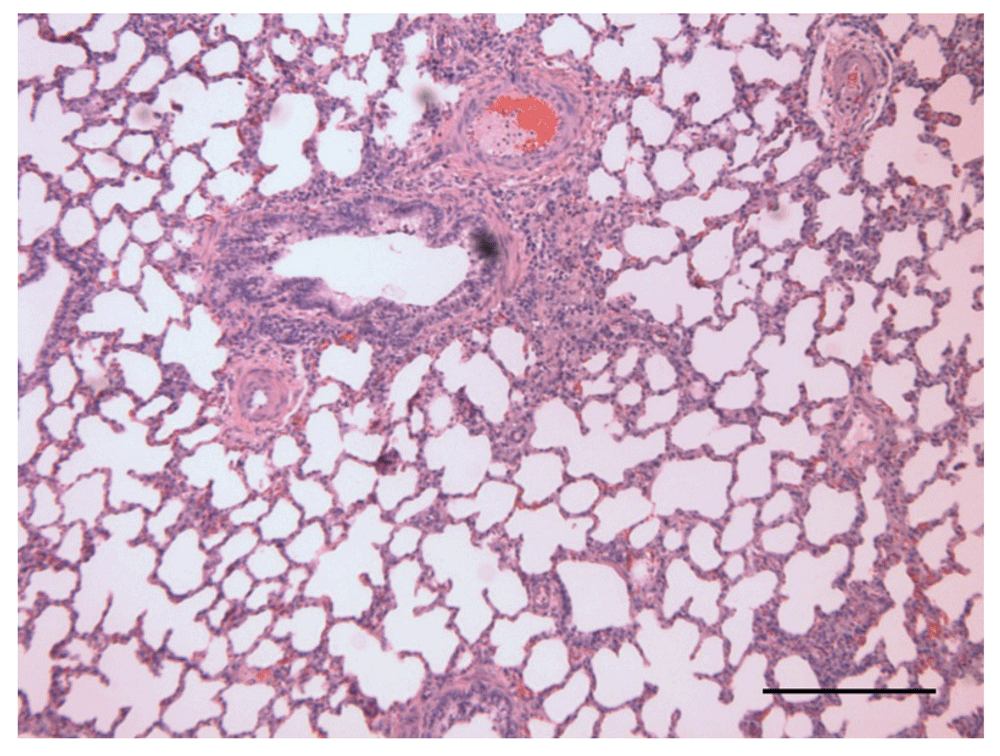
H&E staining. Bar = 0.25 mm.
All calves showed histologic evidence of hepatic congestion, which primarily affected hepatic lobule zones 1 and 2. Two hypoxic calves and one control calf showed hydropic degeneration. Two control calves had hepatic lipidosis. Only one calf, a control (calf 3), had liver microabscesses.
Control calves had greater intestinal permeability to mannitol than the hypoxic calves on Day 0 but had similar intestinal permeability at Day 14 (Figure 3). Control calves tended to have greater permeability to lactulose than hypoxic calves throughout the study. Serum lactulose levels were 0.8 ± 0.4 mg/L greater in the control group than the hypoxic group (P = 0.08). Serum lactulose levels decreased 1.0 ± 0.4 mg/L from Day 0 to Day 14 (P = 0.02). Serum lactulose levels did not significantly vary among sampling time points (P = 0.29).
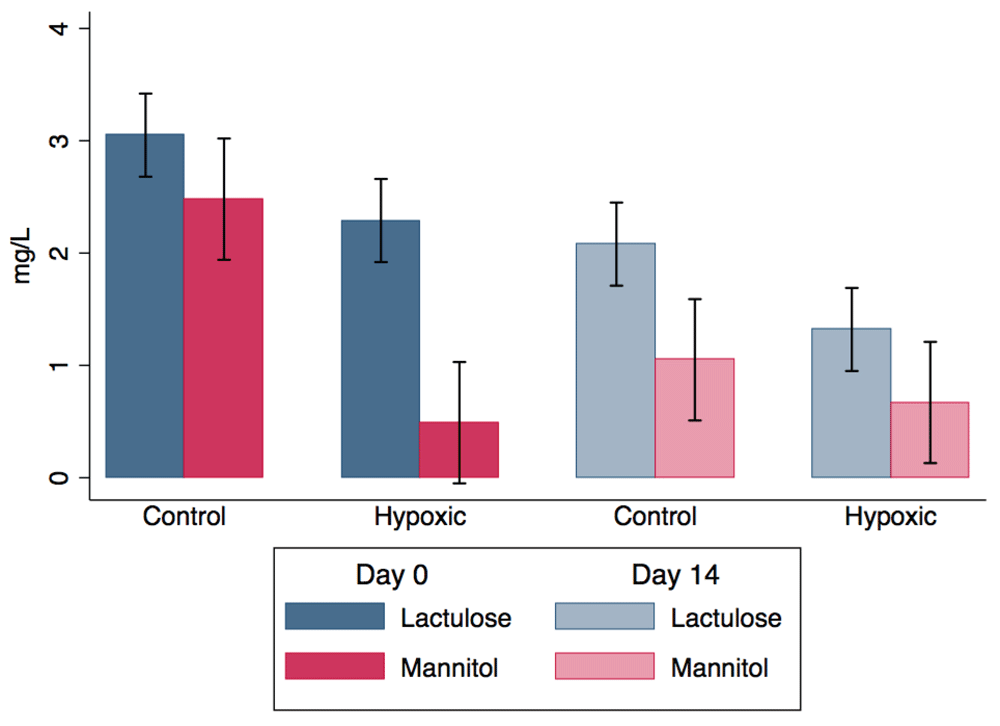
Serum concentrations of lactulose and mannitol in 2-month old Holstein calves at 0 and 14 days of exposure to hypoxic (4,570 m altitude) or normoxic (975 m altitude) conditions.
Serum mannitol levels were 2.0 ± 0.8 mg/L greater in control calves relative to hypoxic calves on Day 0 (P = 0.009). There was a significant interaction between group and Day (P = 0.04). Serum mannitol decreased by 1.4 ± 0.6 mg/L in control calves from Day 0 to Day 14 (P = 0.01), but there was no change in serum mannitol in the hypoxic calves (P = 0.76). There was no difference in serum mannitol between groups at Day 14 (P = 0.61). Mannitol levels tended to decrease by 0.2 ± 0.1 mg/L per hour from administration (P = 0.09).
The serum lactulose to mannitol ratio decreased by 2.7 ± 1.1 from Day 0 to 14 in the hypoxic group (P = 0.01) but there was no change in the ratio between Days 0 and 14 in the control group (P = 0.85). Calves in the hypoxic group had a ratio that was 2.8 ± 0.9 mg/L greater than the control calves on Day 0 (P = 0.003). There was a tendency for an interaction between group and Day (P = 0.10). The ratio did not significantly vary among over time from administration (P = 0.86).
The findings of this study provide preliminary evidence that intestinal inflammation may be associated with pulmonary disease in cattle. Unfortunately, we were unable to test our proposed hypothesis because control calves developed bloody scours. Because of this unforeseen event, however, the findings are all the more notable. The control calves showed a similar increase in mean pulmonary arterial pressure as the calves housed under hypoxic conditions, but they had significantly greater arterial oxygen tensions indicating that the increase in mean pulmonary arterial pressure was not attributable to hypoxia-induced pulmonary hypertension. Furthermore, the two calves that developed bloody scours had gross and microscopic pathology consistent with diffuse alveolar damage, the histologic counterpart of acute lung injury. In concert, these findings provide preliminary evidence that intestinal inflammation may contribute to the development of pulmonary disease in cattle.
Given the small study size, our findings are not robustly supported by statistical analyses. There is, however, considerable supporting evidence for an inter-relationship between the pulmonary and gastrointestinal systems. This is to be expected given that the pulmonary and gastrointestinal systems share a common embryonic origin: the lungs evolved as an outgrowth from the primitive gut. In one study of feedlot cattle, the incidence of acute interstitial pneumonia (AIP) was reported to be 70% greater in pens in which at least one animal had died from a digestive disorder than pens in which digestive disorder death loss did not occur5. There is also accumulating evidence that dietary intervention with probiotics may have a favorable effect on the incidence and recovery of cattle from respiratory diseases6,7. Evidence for a link between inflammatory diseases in the respiratory and gastrointestinal systems in humans is also mounting8. Crohn’s Disease sufferers, for example, are approximately 3-times more likely to die from chronic obstructive pulmonary disease (COPD) than none sufferers9,10. It is plausible that inflammatory mediators released into the circulation by inflamed bowel mucosa triggers a secondary inflammatory event within the lung11.
Inflammatory mediators likely contributed to the rise in mean pulmonary arterial pressure in the control calves through either a direct effect of the pulmonary vasculature or indirectly by inducing diffuse alveolar damage. Alveolar hypoxia was unlikely the primary because arterial oxygen tensions were significantly greater in the controls than the calves housed under hypoxic conditions. Gut-derived gram-negative sepsis likely contributed to the development of pulmonary hypertension in the control calves in our study. Intravenous injection of calves with endotoxin was reported to increase pulmonary arterial resistance and pressure mediated, in part, by prostaglandin F12–15. Most notably, calves with large pressor responses had increased pulmonary arterial wedge pressures, which may have reflected pulmonary venous hypertension and interstitial edema formation14. Furthermore, pulmonary edema was observed on gross and histological examination of calves given heat killed Pseudomonas aeruginosa organisms14 or endotoxin15. Similarly, studies of broiler chickens intravenously16 or intratracheally17 injected with lipopolysaccharide (LPS) reported a significant but short-lived increase in mean pulmonary arterial pressure. In humans, pulmonary hypertension is commonly reported in association with acute lung injury; however, it is unknown if the hypertension is merely a consequence of increased arterial resistance secondary to diffuse alveolar damage, if the increased arterial pressure contributes to the development of alveolar damage, or if they share a common etiology18.
The etiologic agent of the bloody scours was not investigated in our study, but we believe that it was most likely attributable to coccidiosis due to the age of the calves, the positive response to treatment, and the potential for parasite accumulation in the straw bedding. Given that the calves were not housed under identical conditions there are other potential confounding factors, such as environmental temperature and straw bedding that need to be considered; however, other than the development of bloody scours, there is no evidence, to our knowledge, that any of these factors could have contributed to the findings of this study.
In conclusion, we report the development of pulmonary arterial hypertension and diffuse alveolar damage in 2-month old Holstein calves following an acute episode of bloody scours at an altitude of 975 m. The findings of this study provide preliminary evidence that inflammatory gastrointestinal-pulmonary cross-talk may contribute to pulmonary arterial remodeling and hypertension in cattle.
The full UPLC-MS results for mannitol and lactulose for all calves and times of collection is available at: http://dx.doi.org/10.7910/DVN/OU5YC119.
Histology images of the liver and lung for all calves are available at: http://dx.doi.org/10.7910/DVN/FB6GXV20.
Photographs were only taken of the heart, lungs, and intestine of calf 2. They are available at: http://dx.doi.org/10.7910/DVN/TMS3AE21.
The authors would like to thank Dr. Todd Anderson, Director of the The Institute of Environmental and Human Health, Texas Tech University, for the provision of resources and use of the UPLC-MS facilities.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Animal pathology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 11 Oct 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)