Introduction
During the 2000s, several malaria control interventions have been largely adopted and scaled up in endemic countries. These interventions mainly include long lasting insecticidal nets (LLIN), indoor residual spraying (IRS), use of rapid diagnostic tests (RDT) to improve malaria diagnosis and artemisinin-based combination therapy (ACT) as first-line for the treatment of uncomplicated malaria, intermittent preventive treatment of pregnant women (IPTp), intermittent preventive treatment for infants (IPTi), and seasonal malaria chemoprevention (SMC). This considerable deployment of malaria control interventions has largely benefitted from the increase in international funding for the fight against malaria1.
Before being scaled up, the efficacy of control interventions needs to be demonstrated in controlled trials (phase III). These trials consist generally in randomizing individuals or clusters, half receiving the intervention tested, and the other half receiving a control, i.e. a placebo or the best intervention available. Both the intervention and the control are strictly delivered under monitored conditions, in order to preclude biases in the estimate of the efficacy. For any given intervention, especially life-saving interventions, a small number of trials are conducted, because once the efficacy or the superiority of the intervention has been demonstrated it would be unethical to keep providing a less effective intervention to the population, and also because of the important resources needed to conduct such trials. These studies are indispensable to ensure that people receive best interventions available but they poorly depict what will be the effectiveness of the intervention once deployed in real life, for two reasons: (i) phase III trials are conducted in a limited number of settings that don’t encompass all possible field conditions, and (ii) special attention is paid in delivering the intervention during controlled trials, thus rendering ‘ideal’ conditions.
National malaria control programs and donors should nevertheless make sure that the effectiveness of the interventions is confirmed once they have been deployed on the field. As a matter of fact, detecting suboptimal effectiveness of control interventions is critical for policy guidance. This is becoming particularly important since the global budget of the fight against malaria ceased growing1 and thus funding tends to be allocated for most effective interventions. The word “effectiveness” actually encompasses three concepts2,3: the coverage (does the intervention reach the population?), the individual protective effectiveness (does the intervention protect against or treat the disease?), and the community protective effectiveness (does the intervention benefit for others than the very ones who received the intervention?). Surveillance data and ecological studies can measure the overall impact of the control policy but they won’t be helpful in determining whether each intervention taken separately yielded the expected impact since all interventions are usually implemented concomitantly, and because they are influenced by environmental and social factors. Cross-sectional indicator surveys and social sciences studies will provide useful data regarding coverage of interventions and their determinants, but some data may be missing regarding the protective effectiveness (PE) of the interventions.
As mentioned above, it would be unethical - and laborious - to conduct controlled trials in all existing conditions and areas in order to verify the PE of malaria control interventions. Thus, alternative study designs must be applied. The PE can be evaluated (i) by biological studies measuring the bio-efficacy of drugs or insecticides (indirect measurement of the PE) or (ii) by epidemiological surveys yielding a direct measure of the PE of the intervention. The second approach encompasses three major study designs for phase IV assessment of the effectiveness of malaria control interventions, using historical non-compliant controls2: case-control surveys (CCS), cross-sectional surveys (CSS), and cohorts. The stepped-wedge design also allows for the evaluation of the PE, although it sometimes relates to the efficacy (phase III) when the implementation of the intervention is strictly overseen, and sometimes to the effectiveness (phase IV) when the intervention is implemented under field conditions. All these study designs share as a common drawback the possibility of biases: non-compliant controls are not strictly comparable to people having received and using the intervention. Adjusting for socio-demographic variables can reduce but not eliminate biases at the individual level, and adjustment variables can be challenging to identify and to quantify when it comes to the evaluation of the community PE (ecological bias).
In order to assess the disparities between efficacy and effectiveness of malaria control interventions, and the range of PE observed on the field, we conducted a systematic review of the literature on epidemiological studies providing data about the protective effectiveness of control interventions for malaria prevention (CIMP) under field conditions. This study also has as secondary objectives (i) to appreciate what were the study designs most used, (ii) which populations were most surveyed, and (iii) what was the representativeness of these studies.
Methods
The PubMed database was searched for references using an algorithm (provided in Supplementary File 1) looking for (i) keywords related to malaria and (ii) keywords related to control interventions for prevention and (iii) keywords related to study designs that allow for the measure of the effectiveness. Bibliographies of the articles identified were also examined to find additional reports. The search was run for the last time on June 23, 2015.
We intentionally excluded efficacy studies such as controlled trials in the context of phase III assessment, and studies that aimed at measuring indicators such as coverage or factors associated with the uptake of interventions. Studies aiming at measuring the bio-efficacy of interventions using other methods than epidemiological, were also excluded from the database. The present study focuses on intervention for malaria prevention: LLINs, IRS, IPTp, SMC, IPTi, larval source management, and information, education and communication (IEC) campaigns. Given that the use of other insecticides than IRS, such as repellents or mosquito coils, have recently demonstrated an interest in preventing malaria4–6, their PE were also recorded. Articles presenting the effectiveness of IEC regarding prevention behaviours were included. Only articles in English, French, Dutch, or Spanish were considered.
We focused on two outcomes: (i) the measure of the PE against peripheral parasitemia, as measured by RDT and/or blood smears and/or PCR, and (ii) the measure of the PE against occurrence of acute clinical malaria. In studies having investigated other biological or clinical outcomes simultaneously, we recorded preferentially the two outcomes mentioned above. Whenever several PE results were available from a single study (e.g. for different subpopulations), all PE results were retrieved.
On the basis of the objectives of the study disclosed in the title, the abstract and the article, we determined whether the study was aimed at measuring the effectiveness of a CIMP or if this measure was done “accidentally”, e.g. for the purpose of controlling for other associations. The PE was defined as one minus odds ratio (OR) or one minus relative risk (RR), depending on the study design.
Results
Of the 1423 references retrieved, 523 were discarded on the basis of the title; 893 abstracts were checked and 683 of these didn't address the effectiveness of CIMP; seven abstracts could not be accessed (see flow diagram provided in Supplementary File 2). We thus identified 203 papers related to studies that aimed at measuring the effectiveness of CIMP or in which the effectiveness of CIMP was measured but 10 of them could not be accessed. One study was excluded because it focused on travellers and not resident populations of endemic areas; one reference was excluded because of the language; one study was excluded because it evaluated an intervention that was not particularly targeting malaria or mosquito control (cotrimoxazole in HIV positive pregnant women); two studies were excluded because it evaluated a CIMP out-dated (chloroquine chemoprophylaxis in pregnancy); 14 studies were excluded because no OR or RR value was presented and the data disclosed in the article didn’t allow for calculation of the PE of CIMP. Among the remaining 175 references, 13 presented methodological problems incompatible with the inclusion in the present review, such as absence of definition of cases or definition of the exposure to CIMP.
The final review included thus 162 studies. This included 133 (82.1%) studies on bed nets, 37 (22.8%) studies on IPTp, 25 (15.4%) studies on IRS, and 22 (13.6%) studies on other interventions (Figure 1). One third of the studies (52/162, 32.1%) addressed more than one CIMP. Regarding studies' design, 106 (65.4%) were CSS, 29 (17.9%) were CCS, 24 (14.8%) were cohorts, and 3 (1.9%) were stepped wedge (Figure 1).
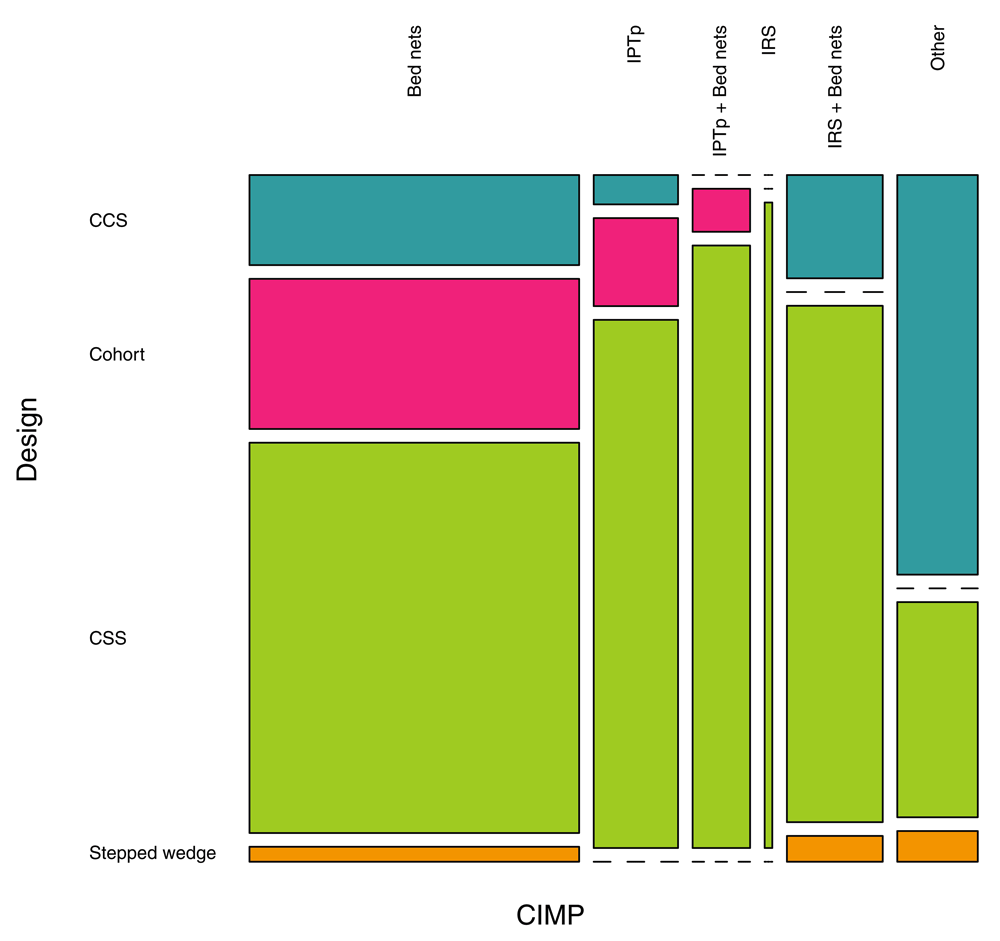
Figure 1. Proportions of CIMPs investigated in the 162 studies included in the review, and the proportions of study designs used.
The number of publications related to the PE of CIMP increased considerably during the years 2000’s and stagnated since 2010 (Figure 2). Two fifths (41.4%) of these studies were not directly aimed at measuring the PE of CIMP.
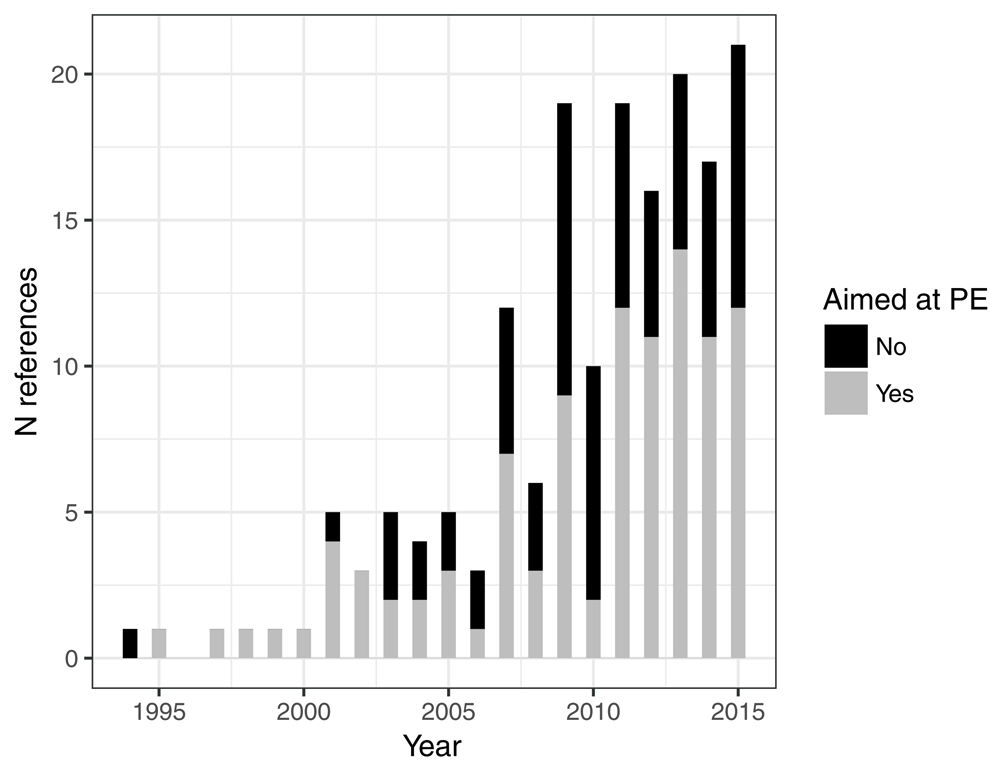
Figure 2. Number of references by year of publication, and the proportion of studies whose aim was the measure of the effectiveness of CIMP.
The last year is truncated since the search was performed in June 2015.
Regarding the representativeness of the studies, two thirds (109/162, 67.3%) were conducted at the district level or below. Only one third of the studies that didn’t investigate the effectiveness of IPTp (43/125, 34.4%) included the whole population while the majority (71/125, 56.8%) included only children. Only 15 of these studies (12.0%) were conducted in the whole population and at a regional level (≥2 districts, province/region, or island) or above (national or multi-country).
In 18.1% (50/276) of the evaluations of PE retrieved or recalculated from the 162 studies included in the review, the association between the malaria outcome and the exposure to the CIMP was not adjusted on other variables (univariate logistic regression, two-by-two tables, etc). Since the adjustment on age is particularly important, we calculated the proportion of studies conducted in a population where the age of the oldest participants was ≥10 years older than the youngest, but for which no adjustment on age was done in the measure of the PE. We found that 38.9% of the studies conducted in such a population with heterogeneous age groups didn’t adjust the calculation of the PE for age.
PE of bed nets
The search retrieved 169 measures of the PE of bed nets in 133 studies (Supplementary File 3). Most of the time (82.2%), the exposure to bed nets was measured at the individual level, but in 23 cases (13.6%) it was done at household level (ownership or proportion of users) and in seven cases (4.1%) at cluster level. The majority of PE measurements involved insecticide-treated nets (ITN) or LLINs (42.0 and 12.4%, respectively) but in an important proportion of cases the definition of bed nets didn’t include impregnation or not (42.6%). In some instances (2.9%), the measurement was specifically done for non-impregnated bed nets (NIBN). Most PE evaluations used the Plasmodium infection as outcome (56.8%), especially CSS that accounted for 62.7% of study designs (Figure 3), or clinical malaria (31.9%), especially CCS that accounted for 20.1% of study designs; some used an obstetrical outcome (7.1%), and a few ones used the mortality as outcome (4.1%). Cohorts represented 15.4% of study designs and there were only three stepped-wedge designs (1.8%). More than a half of PE results (58.0%) were obtained from paediatric populations and 27.8% considered the whole population; the other studies (14.2%) were conducted on women of childbearing age.
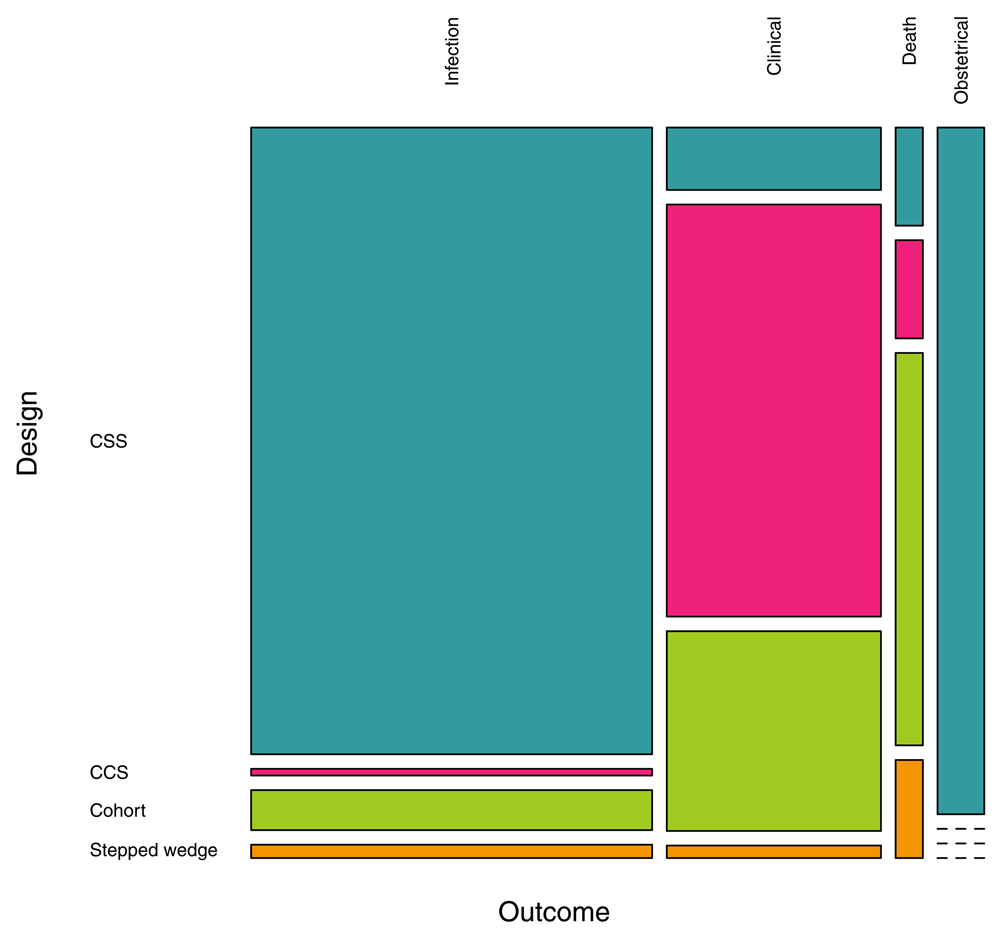
Figure 3. Proportions of study designs and outcomes investigated for the evaluation of PE of bed nets.
Most of the results (60.7%) demonstrated a significant PE from bed nets use. However, 38.2% of results were not significant (Figure 4, Figure 5 and Supplementary File 3). In 14.2% of cases, the PE value was negative, i.e. a trend towards a risk increased, and 1.2% of results showed a risk significantly increased. The median PE was 36.0% (interquartile range [IQR] 14.0–54.0%), and this differed only marginally according to CIMP definition: median PE was 39.8% (IQR 20.2–50.3%) for LLINs, 30.0% (IQR 16.5–52.0%) for ITNs, 51.0% (IQR 35.0–51.0%) for NIBNs, and 36.0 (IQR 13.8–54.6%) for bed nets without further precision of their impregnation.
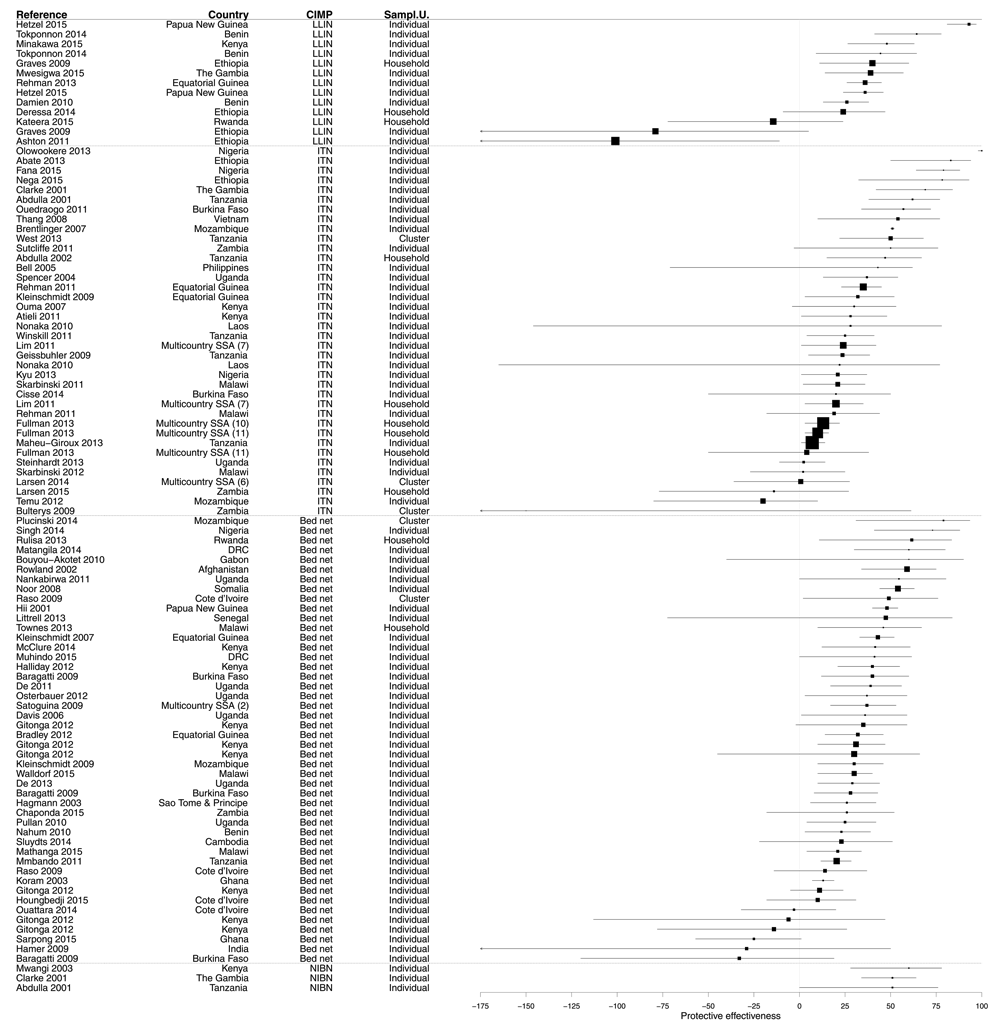
Figure 4. Forest plot of evaluations of PE of bed nets against infection.
Results without CI are not displayed. Box size is proportional to the sample size.
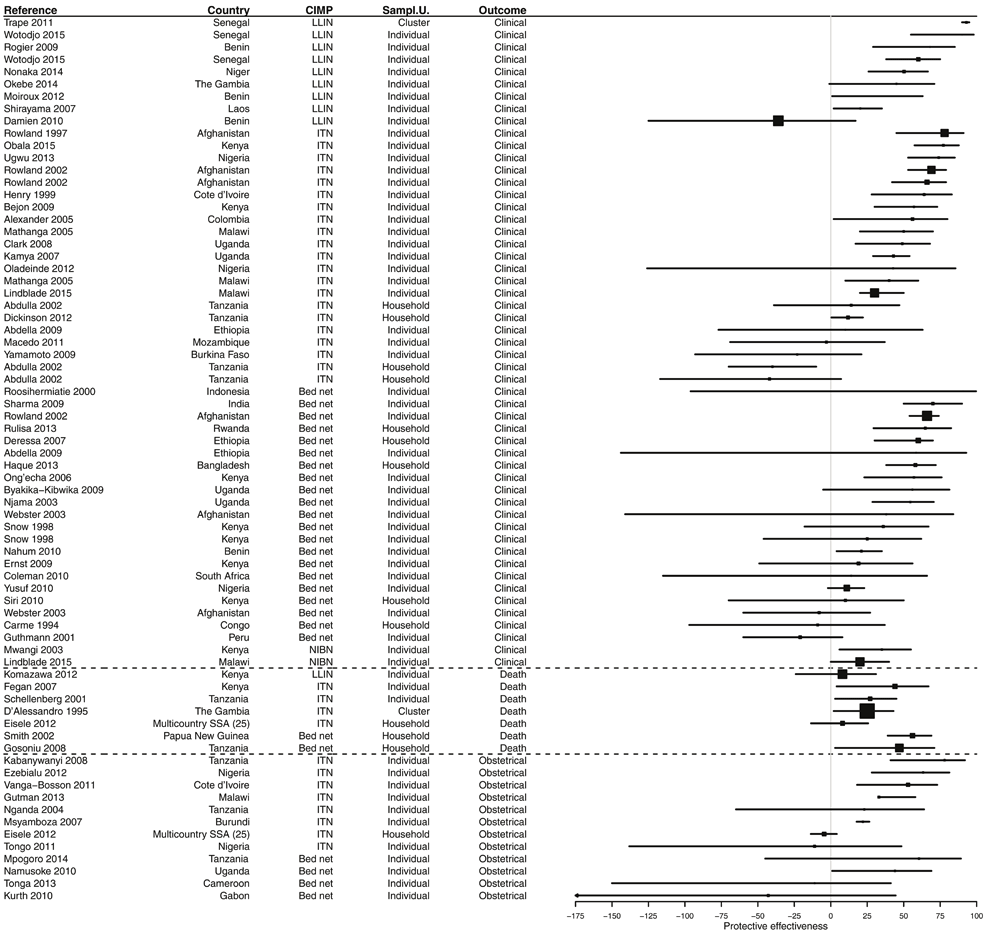
Figure 5. Forest plot of evaluations of PE of bed nets against other outcomes than infection.
Results without CI are not displayed. Box size is proportional to the sample size.
PE of IRS
The search retrieved 32 measures of the PE of IRS in 25 studies (Supplementary File 4). CSS survey design was largely predominant (90.6%) and three PE evaluations from CCS (9.4%) were observed. A third of studies (34.4%) considered the whole population while 59.4% were obtained from paediatric populations and 6.3% from women of childbearing age. Most of the time (78.1%), the exposure to bed nets was measured at the household level, but in seven cases (21.9%) it was measured at cluster level. Only 21.9% of PE measurements of IRS considered recent spraying (≤6 months before the survey or delay since last IRS round in months), and the rest considered IRS ‘last round’, ‘last year’, or even ‘ever’. Most PE evaluations used the Plasmodium infection as outcome (87.5%) and the rest considered clinical malaria (12.5%).
Half of results demonstrated a significant PE of IRS (median 28.5%, IQR 8.8–47.3%), but 43.8% of results were not significant and 6.2% of results showed a risk significantly increased (Figure 6 and Supplementary File 4). The PE value was positive in more than three results out of four (78.1%). Median PEs were comparable when considering recent (20.0%, IQR -2.5–41.0%) or older spraying (32.0%, IQR 9.0–46.0%).
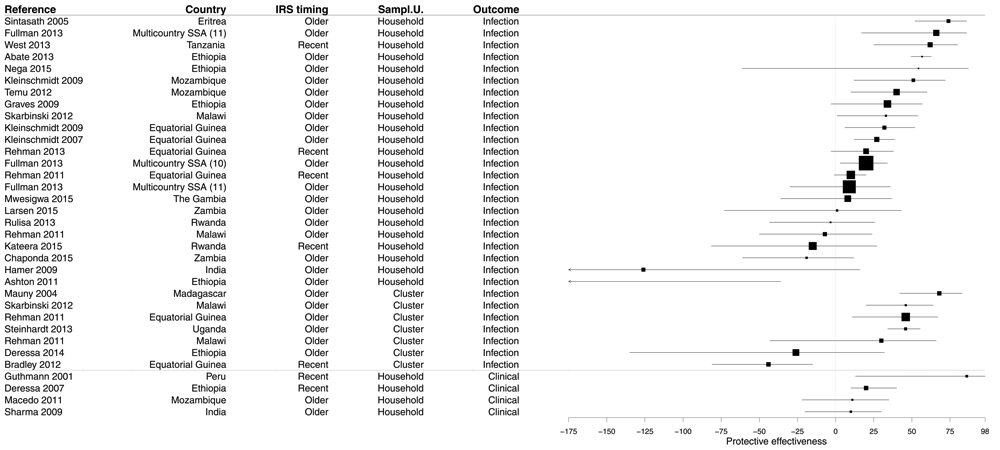
Figure 6. Forest plot of evaluations of PE of IRS.
Box size is proportional to the sample size. Recent spraying: ≤6 months before the survey or delay since last IRS round in months.
PE of concurrent exposure to ITN and IRS
Our systematic search allowed us identifying only five studies and eight results about the PE of concurrent exposure to ITN and IRS (Table 1). Two results compared the exposure to both interventions versus IRS only, and the six other compared the exposure to both interventions versus no intervention. All study designs were CSS and all evaluated the effectiveness of ITN or LLIN against infection in children.
Table 1. PE of the exposure to IRS and bed nets in decreasing order, by reference group for PE measurement.
*: Indicates significant result as compared with exposure to one CIMP only. MC: Multi-country study stratified by transmission (low-medium-high), the number between brackets indicates the number of countries. †: PE versus IRS only. ‡: PE versus no ITN and no IRS.
| Country | PE of ITN + IRS
(%) [95%CI] | PE‡ of ITN
(Supplementary File 2) | PE‡ of IRS
(Supplementary File 3) | Reference |
|---|
| Mozambique | 37 [21;50]*† | 30 [10;46]* | 51 [12;72]* | Kleinschmidt 200931 |
| Equatorial Guinea | 35 [2;57]*† | 36 [26;45]* | 20 [-3;38] | Rehman 201311 |
| Equatorial Guinea | 29 [14;41]*† | 32 [3;52]* | 32 [6;52]* | Kleinschmidt 200931 |
| MC (10) – Med. transm. | 53 [37;67]*‡ | 13 [3;22]* | 20 [3;34]* | Fullman 201342 |
| Mozambique | 50 [30;70]‡ | -20 [-80;10] | 40 [10;60]* | Temu 201246 |
| MC (11) – Low transm. | 33 [-33;70]‡ | 4 [-50;38] | 66 [17;86]* | Fullman 201342 |
| MC (11) – High transm. | 31 [11;47]‡ | 10 [3;16]* | 9 [-30;36] | Fullman 201342 |
| Malawi | 19 [-19;44]‡ | 2 [-27;25] | 33 [1;54]* | Skarbinski 201245 |
Four out of eight results demonstrated a significant added PE of using both interventions as compared with one of these two CIMP only; in these studies (or sub-studies) ITN and IRS alone had both demonstrated significant PE –the PE of IRS in the study of Rehman et al. is borderline. In the other four (sub-)studies, one of the two CIMP had failed to demonstrate a significant PE and the exposure to both interventions either showed an added protection but non-significant as compared with one CIMP only or provided a PE inferior to the PE of IRS only.
PE of IPTp
Our search retrieved 40 measures of the PE of IPTp using sulfadoxine-pyrimethamine (SP) in 37 studies (Supplementary File 5). Among these 40 results, 16 (40.0%) compared any regimen versus no SP dose, 13 (32.5%) compared the standard regimen versus no IPTp, and the remaining 11 (27.5%) compared the standard regimen versus substandard regimen. Most PE evaluations used an obstetrical or neonatal (e.g. low birth weight) outcome only (45.0%) or an outcome considering an obstetrical event or a maternal peripheral parasitemia (7.5%). The detection of Plasmodium in the mother’s blood was used as the outcome in 37.5%; three results (7.5%) had evaluated clinical malaria, and one used the mortality as outcome (2.5%). CSS represented 85.0% of study designs, cohorts 10.0% and there were only two CCS (5.0%). Most results (90.0%) were obtained from mothers, usually pregnant women at antenatal consultation and/or women at delivery units, but 10.0% considered paediatric populations (neonates or infants). The vast majority of studies were conducted at the district level or below (85.0%).
Most results demonstrated a significant PE of IPTp (median PE 49.0%, IQR 23.0–67.3%), but 32.5% of results were not significant and 5.0% of results showed a risk significantly increased (Figure 7 and Supplementary File 5). Median PE was 24.7% (IQR 4.0–70.0%) in studies evaluating standard IPTp regimen versus no IPTp, 50.5% (IQR 30.0–65.0%) in studies comparing any IPTp regimen versus no IPTp, and 50.0% (IQR 35.8–65.4%) in studies evaluating standard IPTp regimen versus substandard IPTp regimen. These values were comparable between studies evaluating the PE of IPTp against infection (median PE 43.0, IQR 7.0–78.5%) and studies evaluating the PE of IPTp against obstetrical outcomes (median PE 53.5, IQR 33.3–66.2%).
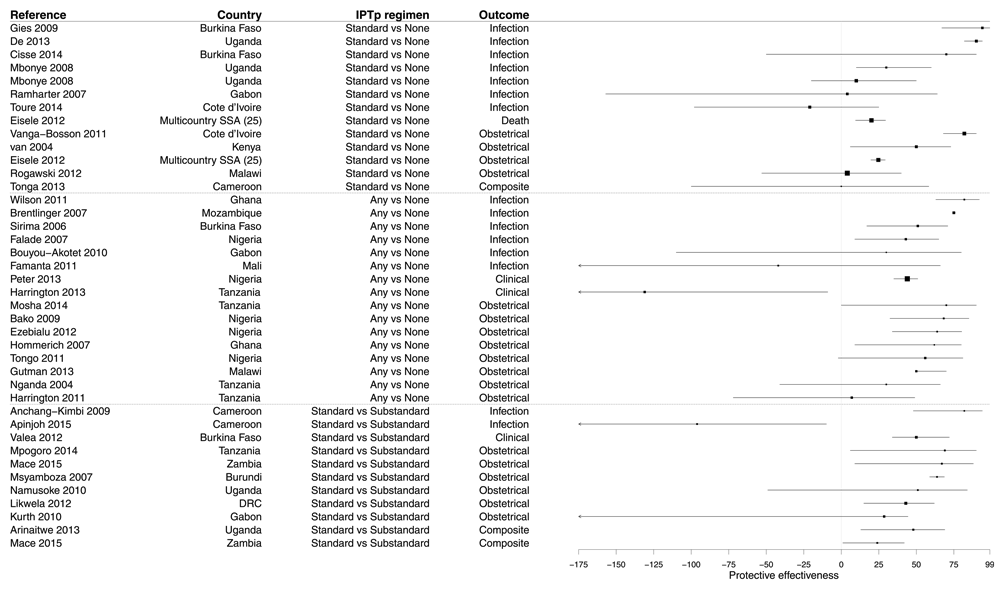
Figure 7. Forest plot of evaluations of PE of IPTp.
Box size is proportional to the sample size.
PE of the domestic use of insecticides
Our systematic search identified 20 evaluations, from 16 studies (Supplementary File 6), of the PE of the use of other insecticides than IRS, including coils (45.0%), sprays (30.0%), and repellents (10.0%). In the remaining 15.0% of cases, the PE of two or three of these formulations together was evaluated. The vast majority of these results came from CCS evaluating clinical outcomes (15/20, 80.0%), and the four other results came from CSS evaluating clinical (1/20) infection (1/20), or obstetrical (2/20) outcomes. The majority of these studies were conducted in paediatric populations (65.0%), some in the whole population (20.0%) and the remaining 15.0% among adult women.
Overall the PE of these insecticides was demonstrated in only four studies, whatever the formulation in coils, sprays, or repellents, and most (70.0%) results were non-significant (Figure 8 and Supplementary File 6). The median PE was 19.1% (IQR -21.0–38.5%).
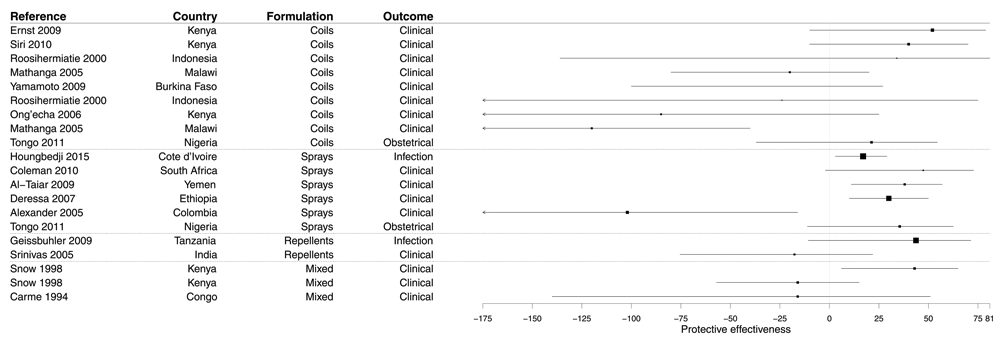
Figure 8. Forest plot of evaluations of PE of domestic use of insecticides.
Box size is proportional to the sample size.
Other interventions
Our search retrieved only one study aiming to evaluate the PE of IPTi. It was a CCS conducted among infants in Tanzania and its main result was that the PE against occurrence of clinical malaria cases was 18% and not significant (95% CI -129–71%165).
We identified two studies evaluating the PE of larviciding programs by comparing clusters receiving the intervention and clusters that were not treated, either in a CSS design applied in under-fives38 or in a stepped-wedge design encompassing all age groups43. Both studies showed a significant PE of larviciding against infection by Plasmodium of 72% (20–90%) and 21% (7–34%) respectively.
In this review, no study assessing the PE of IEC interventions on malaria indicators has been found, but we found two countrywide CSS evaluating the effectiveness of the exposure to IEC programs on bed net (or ITN) use. One was conducted in adult population of Cameroon166 and the other one in adult women of Zambia167. These two studies showed that being exposed to IEC interventions was associated with an increase in bed net use (OR 1.48, 95% CI 1.18–1.86, and OR 1.62, 95% CI 1.28–2.04, respectively) by logistic regression with propensity score matching.
Finally, we found one study having evaluated the PE of availability of a village health worker trained for malaria management against Plasmodium infection through a CSS conducted among all age groups in a province of the Philippines27. They found a significant PE of 74% (16–92%).
Dataset 1.Data underlying the results presented in this systematic review.
Discussion
This review showed that the efforts made for the evaluation of the effectiveness is increasing with time, in parallel with the global funding available for malaria control. Nevertheless, the number of published studies about the effectiveness of CIMPs seems to be stagnating since 2010. This could hinder the progress towards more cost-effective control policies, as the strategy should be locally adapted depending on data about the effectiveness of CIMP.
Overall, there is a sense of a low representativeness of the studies. Only one third of the studies were conducted at a large scale, and only one third included all ages and genders; only one out of eight had both features. Several CIMPs target the whole population of a region or a country, e.g. IRS or universal distributions of LLINs. When evaluating the PE of such CIMP, it’s crucial not to leave aside a part of the population since the effectiveness of CIMP may vary depending on transmission or between age groups for example67,168,169. On the contrary, nearly 40% of studies conducted across age groups didn’t include the age in regression models while age influences both malaria outcomes (e.g. probability to be infected) and CIMP coverage170. In order to yield unbiased evaluation of the PE of a CIMP, it is critical to adjust the measure of the association of malaria outcome and exposure to CIMP for age, as well as for other variables known to influence the outcome (e.g. socio-economic status, parity, rural or urban area) and to take into account the intra-cluster correlation in multi-stage sample designs. Moreover, several studies conducted at a large scale didn’t stratify the analysis. Since local features of malaria transmission or cultural behaviours may affect (or enhance) the PE of CIMPs, omitting stratified analysis precludes the identification of clusters where the effectiveness of CIMPs was suboptimal.
On the other hand, the multiplicity of local evaluations of the PE of CIMP offers an appreciation of the diversity of local conditions. Certain studies revealed that the PE was largely above what had been demonstrated in efficacy trials; this can result from biases inherent to observational studies, but it’s also possible that local conditions favour the effectiveness of CIMP, e.g. the PE of LLINs is expected to be especially high where vector populations exclusively bite indoor and late at night. Conversely, many studies failed to demonstrate the PE of CIMP studied or showed that it was lower than expected. This is where the interest of these surveys stands, for it urges policy makers and their research partners to investigate the causes of this failure and to propose alternative control interventions. This is also why we didn’t conduct a meta-analysis on the data retrieved in this review. Besides this, various meta-analyses of CIMP already exist, either reviewing efficacy studies only169,168,171, or mixed both efficacy and effectiveness studies172.
This review presents several limitations, including the search in one database only, the limits in languages considered (although one reference only was discarded for this reason), and the incomplete access to articles. This review is thus probably not exhaustive but it intends to be largely representative of effectiveness studies. On purpose, we didn’t include meta-analyses of the studies included as the overall objective was to get a sense of what kind of studies had been done, and to be strictly descriptive on the results obtained. Our take-home message is not so much that MCI are effective on average, but that their effectiveness might be locally lower -or higher- than what is expected by efficacy studies.
Bed nets
Overall, the measures of the PE of bed nets demonstrated a fair effectiveness of this CIMP, even often above the protective efficacy measured in controlled trials. This phenomenon can be attributed to local features of malaria transmission (e.g. intensity of transmission, vector biting behaviour, vector sensitivity to insecticides, or human behaviour), or to differences in outcomes used in efficacy trials (often clinical outcomes) versus those used in effectiveness studies (often the infection by Plasmodium parasites), or to differences in the definition of the exposure to bed nets. For example, it has been shown that in low transmission areas LLIN perform better and/or parasitemia is a better indicator of LLIN performance37,168. Controlled trials performed in areas of high transmission (e.g. two meta-analyses of studies conducted in such areas showed protective efficacies of 13%168 and 17%173, respectively) have shown lower protective efficacy than those conducted in low transmission areas, e.g. in Kenyan highlands (protective efficacy 63%,174) or in Pakistan (protective efficacy 43%,175).
Various definitions of bed net exposure have been used throughout the studies included in the present review: type of bed net (LLIN, ITN, bed net without further definition of impregnation but sometimes in areas where most bed nets are actually impregnated, or NIBN), intensity of exposure (ownership, bed net/person ratio, use the previous night, or regular use), and level of measure of exposure (individual, household, cluster). Surprisingly, in our review, it seems that the definition of exposure to bed nets does not impacts importantly the measure of the PE. Therefore, it is possible that evaluations of the PE of LLINs or ITNs yield an estimation of the effectiveness provided by the physical barrier against vectors’ bites and underestimate the community effect offered by insecticides impregnation.
IRS
Effectiveness studies generally verified the PE of IRS, whether at community or household level. It’s complicated to compare those results with efficacy studies since those are relatively scarce. Indeed, IRS has been deployed before the requirement of a demonstration of efficacy of MCI through randomized controlled trials. A meta-analysis from 13 efficacy and effectiveness studies conducted in 11 countries measured a pooled household-level and community-level protective efficacy of 62%172, but other controlled trials showed more limited protective efficacy at the community level, e.g. in India where it was 28%169,176 and in Nigeria during the wet season where it was 26%169,177. Controlled trials even sometimes showed very limited efficacy like in Nigeria during the dry season or in Tanzania (protective efficacy 6%169,177,178).
As for bed nets, the vectors’ biting behaviour, their sensitivity to insecticides, and the endemicity of malaria are expected to influence most the PE of IRS. These factors should be investigated in areas where IRS fails to demonstrate its effectiveness in order to guide local malaria control policies.
Concurrent exposure to LLIN and IRS
Overall effectiveness studies plea for the combination of these two vector control interventions since it seems that all studies finding significant PE of the two CIMP separately also found a significant added PE in people benefitting from both CIMP simultaneously. On the contrary, in studies where at least one CIMP failed to demonstrate a significant PE, the added value of using LLIN in a household having received IRS also failed to be proven. Results from randomized controlled trials are more balanced: some did show an additional protection offered by IRS over LLIN only179,180, some did not181–183. Overall, evidence of additional protection of the combination against malaria remains inconclusive184.
IPTp
Most studies aiming to evaluate the effectiveness of IPTp were conducted at small scale, usually in one or two hospitals. Nevertheless, some studies were conducted at a larger scale and even stratified by regions, e.g. a study conducted in 3 regions of the Democratic Republic of Congo showed that, in one regions, the effectiveness of IPTp against low birth weight was affected while it was preserved in the 2 other regions161. Geographical stratification can thus detect an inhomogeneity in the PE that can reflect, for example, local parasitological resistance to SP. This resistance is the major cause to be investigated for policy guidance.
An important limitation of the present review is that IPTp aims at reducing malaria burden in terms of maternal and neonatal morbidity and mortality; obstetrical outcomes are therefore more adapted for the evaluation of the PE of IPTp than maternal peripheral parasitemia or acute clinical episodes of malaria –that we prioritized in our review. Similarly these outcomes were often considered as secondary in efficacy controlled trials. Nevertheless one meta-analysis of three studies conducted in two countries measured a pooled protective efficacy of IPTp against maternal peripheral parasitemia of 55%185 and another trial demonstrated a protective efficacy of 64%186. Besides this, the efficacy of IPTp has been evaluated against several obstetrical outcomes, including low birth weight (significant protective efficacy of 29%185), placental parasitemia (significant protective efficacy of 52%185), maternal anaemia (significant protective efficacy of 10%185), perinatal mortality (non-significant protective efficacy of 22%187), or stillbirth (non-significant protective efficacy of 4%187).
Other interventions
The PE of the use of insecticides was seldom demonstrated, despite the possibility of a socioeconomic bias that would be expected to increase their PE. Further studies will have to be conducted in order to verify that their efficacy translate into effectiveness under field conditions if they are adopted by policy makers.
Our review identified no study having tried to evaluate the effectiveness of IEC interventions against clinical or biological malaria indicators, and only two that demonstrated the effectiveness of IEC programs on bed net coverage. Unfortunately, the uniqueness of media messages and cultural features in these studies preclude the extrapolation of their results. Generally few studies have evaluated the effectiveness of IEC intervention, not only for malaria188. This reflects the rarity of phase III studies aiming at demonstrating the efficacy of IEC interventions on epidemiological indices. This paucity of information is surprising given the popularity of IEC programs in public health.
In 2013, IPTi had been adopted by one country only1, which explains that we found only one study evaluating its PE. More surprisingly, SMC has been adopted by six countries1 but the PE of this CIMP has not been evaluated yet. The small number of studies regarding SMC, IEC or larviciding hinders the interpretation of these results.
Conclusions
This review shows that there is an increasing interest in measuring the PE of CIMPs. Most studies confirmed the PE of the CIMPs that they were evaluating, but an important part yielded a ‘negative’ PE and/or non-significant confidence interval. In this case, complementary investigations are needed in order to confirm the existence of a problem in the effectiveness of the CIMP and to propose alternative control measures if necessary.
A frequent feature of the studies included in this review was the low geographical representativeness and/or the low representativeness in the population studied. Conversely the analyses of large samples were not systematically stratified by subpopulations. We believe that such investigations need to zoom out (encompass a large population) and to zoom in (stratify by subpopulations) to get a complete picture evaluating the effectiveness of CIMPs.
To evaluate properly the PE of a CIMP we recommend to pay attention to the following points: (i) encompass all age groups and genders, except for targeted interventions such as IPTp or IPTi, (ii) sample all geographical and/or cultural patterns, (iii) stratify the evaluation of the PE by subgroups, (iv) adjust for socio-demographic variables that are associated with the outcomes and at least adjust for age and gender if the population sampled is not homogeneous in this regard.
Abbreviations
ACT: artemisinin-based combination therapy, CCS: case-control survey, CSS: cross-sectional survey, CIMP: control intervention for malaria prevention, IEC: information, education and communication, IPTi: intermittent preventive treatment for infants, IPTp: intermittent preventive treatment of pregnant women, IQR: interquartile range, ITN: insecticide-treated nets, IRS: indoor residual spraying, LLIN: long lasting insecticidal nets, NIBN: non-impregnated bed nets, RDT: rapid diagnostic tests, SMC: seasonal malaria chemoprevention; SP: sulfadoxine-pyrimethamine
Data availability
Dataset 1: Data underlying the results presented in this systematic review. DOI, 10.5256/f1000research.12952.d182415189.
Competing interests
No competing interests were disclosed.
Grant information
This research was supported by the Institut Pasteur de Madagascar.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
We thank the Institut Pasteur de Madagascar, and in particular the Malaria Research Unit, for their helpfulness and their sympathy.
Faculty Opinions recommendedReferences
- 1.
WHO Global Malaria Programme: World Malaria Report 2014. Geneva; 2014. Reference Source
- 2.
Lengeler C, Snow RW:
From efficacy to effectiveness: insecticide-treated bednets in Africa.
Bull World Health Organ.
1996; 74(3): 325–332. PubMed Abstract
| Free Full Text
- 3.
WHO Global Malaria Programme: Malaria Control in Humanitarian Emergencies: An Inter-Agency Field Handbook. (Second Edition), Geneva: World Health Organization; 2013. Reference Source
- 4.
Hill N, Zhou HN, Wang P, et al.:
A household randomized, controlled trial of the efficacy of 0.03% transfluthrin coils alone and in combination with long-lasting insecticidal nets on the incidence of Plasmodium falciparum and Plasmodium vivax malaria in Western Yunnan Province, China.
Malar J.
2014; 13: 208. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 5.
Dadzie S, Boakye D, Asoala V, et al.:
A community-wide study of malaria reduction: Evaluating efficacy and user-acceptance of a low-cost repellent in Northern Ghana.
Am J Trop Med Hyg.
2013; 88(2): 309–314. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 6.
Deressa W, Yihdego YY, Kebede Z, et al.:
Effect of combining mosquito repellent and insecticide treated net on malaria prevalence in Southern Ethiopia: a cluster-randomised trial.
Parasit Vectors.
2014; 7: 132. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 7.
Tokponnon FT, Ogouyémi AH, Sissinto Y, et al.:
Impact of long-lasting, insecticidal nets on anaemia and prevalence of Plasmodium falciparum among children under five years in areas with highly resistant malaria vectors.
Malar J.
2014; 13: 76. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 8.
Minakawa N, Kongere JO, Dida GO, et al.:
Sleeping on the floor decreases insecticide treated bed net use and increases risk of malaria in children under 5 years of age in Mbita District, Kenya.
Parasitology.
2015; 142(12): 1–7. PubMed Abstract
| Publisher Full Text
- 9.
Graves PM, Richards FO, Ngondi J, et al.:
Individual, household and environmental risk factors for malaria infection in Amhara, Oromia and SNNP regions of Ethiopia.
Trans R Soc Trop Med Hyg.
2009; 103(12): 1211–1220. PubMed Abstract
| Publisher Full Text
- 10.
Mwesigwa J, Okebe J, Affara M, et al.:
On-going malaria transmission in The Gambia despite high coverage of control interventions: a nationwide cross-sectional survey.
Malar J.
2015; 14: 314. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 11.
Rehman AM, Mann AG, Schwabe C, et al.:
Five years of malaria control in the continental region, Equatorial Guinea.
Malar J.
2013; 12: 154. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 12.
Damien GB, Djènontin A, Rogier C, et al.:
Malaria infection and disease in an area with pyrethroid-resistant vectors in southern Benin.
Malar J.
2010; 9: 380. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 13.
Kateera F, Mens PF, Hakizimana E, et al.:
Malaria parasite carriage and risk determinants in a rural population: a malariometric survey in Rwanda.
Malar J.
2015; 14: 16. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 14.
Ashton RA, Kefyalew T, Tesfaye G, et al.:
School-based surveys of malaria in Oromia Regional State, Ethiopia: a rapid survey method for malaria in low transmission settings.
Malar J.
2011; 10: 25. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 15.
Olowookere SA, Adeleke NA, Abioye-Kuteyi EA, et al.:
Use of insecticide treated net and malaria preventive education: effect on malaria parasitemia among people living with AIDS in Nigeria, a cross-sectional study.
Asia Pac Fam Med.
2013; 12(1): 2. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 16.
Abate A, Degarege A, Erko B:
Community knowledge, attitude and practice about malaria in a low endemic setting of Shewa Robit Town, northeastern Ethiopia.
BMC Public Health.
2013; 13: 312. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 17.
Fana SA, Bunza MD, Anka SA, et al.:
Prevalence and risk factors associated with malaria infection among pregnant women in a semi-urban community of north-western Nigeria.
Infect Dis Poverty.
2015; 4: 24. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 18.
Nega D, Dana D, Tefera T, et al.:
Prevalence and predictors of asymptomatic malaria parasitemia among pregnant women in the rural surroundings of Arbaminch Town, South Ethiopia.
PLoS One.
2015; 10(4): e0123630. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 19.
Clarke SE, Bøgh C, Brown RC, et al.:
Do untreated bednets protect against malaria?
Trans R Soc Trop Med Hyg.
2001; 95(5): 457–462. PubMed Abstract
| Publisher Full Text
- 20.
Abdulla S, Schellenberg JA, Nathan R, et al.:
Impact on malaria morbidity of a programme supplying insecticide treated nets in children aged under 2 years in Tanzania: community cross sectional study.
Br Med J.
2001; 322(7281): 270–273. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 21.
Ouédraogo CM, Nébié G, Sawadogo L, et al.:
[Study of factors favouring the occurrence of Plasmodium falciparum in pregnant women in the health district of Bogodogo].
J Gynecol Obstet Biol Reprod (Paris).
2011; 40(6): 529–534. PubMed Abstract
| Publisher Full Text
- 22.
Thang ND, Erhart A, Speybroeck N, et al.:
Malaria in central Vietnam: analysis of risk factors by multivariate analysis and classification tree models.
Malar J.
2008; 7: 28. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 23.
Brentlinger PE, Montoya P, Rojas AJ, et al.:
Prevalence and predictors of maternal peripheral malaria parasitemia in central Mozambique.
Am J Trop Med Hyg.
2007; 77(2): 228–234. PubMed Abstract
- 24.
West PA, Protopopoff N, Rowland M, et al.:
Malaria risk factors in North West Tanzania: the effect of spraying, nets and wealth.
PLoS One.
2013; 8(6): e65787. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 25.
Sutcliffe CG, Kobayashi T, Hamapumbu H, et al.:
Changing individual-level risk factors for malaria with declining transmission in southern Zambia: a cross-sectional study.
Malar J.
2011; 10: 324. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 26.
Abdulla S, Schellenberg JR, Mukasa O, et al.:
Usefulness of a dispensary-based case-control study for assessing morbidity impact of a treated net programme.
Int J Epidemiol.
2002; 31(1): 175–180. PubMed Abstract
| Publisher Full Text
- 27.
Bell D, Go R, Miguel C, et al.:
Unequal treatment access and malaria risk in a community-based intervention program in the Philippines.
Southeast Asian J Trop Med Public Health.
2005; 36(3): 578–586. PubMed Abstract
- 28.
Maketa V, Mavoko HM, da Luz RI, et al.:
The relationship between Plasmodium infection, anaemia and nutritional status in asymptomatic children aged under five years living in stable transmission zones in Kinshasa, Democratic Republic of Congo.
Malar J.
2015; 14: 83. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 29.
Spencer S, Grant AD, Piola P, et al.:
Malaria in camps for internally-displaced persons in Uganda: Evaluation of an insecticide-treated bednet distribution programme.
Trans R Soc Trop Med Hyg.
2004; 98(12): 719–727. PubMed Abstract
| Publisher Full Text
- 30.
Rehman AM, Coleman M, Schwabe C, et al.:
How much does malaria vector control quality matter: the epidemiological impact of holed nets and inadequate indoor residual spraying.
PLoS One.
2011; 6(4): e19205. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 31.
Kleinschmidt I, Schwabe C, Shiva M, et al.:
Combining indoor residual spraying and insecticide-treated net interventions.
Am J Trop Med Hyg.
2009; 81(3): 519–524. PubMed Abstract
| Free Full Text
- 32.
Ouma P, van Eijk AM, Hamel MJ, et al.:
Malaria and anaemia among pregnant women at first antenatal clinic visit in Kisumu, western Kenya.
Trop Med Int Health.
2007; 12(12): 1515–1523. PubMed Abstract
| Publisher Full Text
- 33.
Atieli HE, Zhou G, Afrane Y, et al.:
Insecticide-treated net (ITN) ownership, usage, and malaria transmission in the highlands of western Kenya.
Parasit Vectors.
2011; 4: 113. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 34.
Nonaka D, Laimanivong S, Kobayashi J, et al.:
Is staying overnight in a farming hut a risk factor for malaria infection in a setting with insecticide-treated bed nets in rural Laos?
Malar J.
2010; 9: 372. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 35.
Winskill P, Rowland M, Mtove G, et al.:
Malaria risk factors in north-east Tanzania.
Malar J.
2011; 10: 98. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 36.
D’Alessandro U, Olaleye BO, McGuire W, et al.:
Mortality and morbidity from malaria in Gambian children after introduction of an impregnated bednet programme.
Lancet.
1995; 345(8948): 479–483. PubMed Abstract
| Publisher Full Text
- 37.
Lim SS, Fullman N, Stokes A, et al.:
Net benefits: a multicountry analysis of observational data examining associations between insecticide-treated mosquito nets and health outcomes.
PLoS Med.
2011; 8(9): e1001091. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 38.
Geissbühler Y, Kannady K, Chaki PP, et al.:
Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in urban Dar Es Salaam, Tanzania.
PLoS One.
2009; 4(3): e5107. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 39.
Kyu HH, Georgiades K, Shannon HS, et al.:
Evaluation of the association between long-lasting insecticidal nets mass distribution campaigns and child malaria in Nigeria.
Malar J.
2013; 12: 14. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 40.
Skarbinski J, Mwandama D, Luka M, et al.:
Impact of health facility-based insecticide treated bednet distribution in Malawi: progress and challenges towards achieving universal coverage.
PLoS One.
2011; 6(7): e21995. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 41.
Cisse M, Sangare I, Lougue G, et al.:
Prevalence and risk factors for Plasmodium falciparum malaria in pregnant women attending antenatal clinic in Bobo-Dioulasso (Burkina Faso).
BMC Infect Dis.
2014; 14: 631. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 42.
Fullman N, Burstein R, Lim SS, et al.:
Nets, spray or both? The effectiveness of insecticide-treated nets and indoor residual spraying in reducing malaria morbidity and child mortality in sub-Saharan Africa.
Malar J.
2013; 12: 62. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 43.
Maheu-Giroux M, Castro MC:
Impact of Community-Based Larviciding on the Prevalence of Malaria Infection in Dar es Salaam, Tanzania.
PLoS One.
2013; 8(8): e71638. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 44.
Steinhardt LC, Yeka A, Nasr S, et al.:
The effect of indoor residual spraying on malaria and anemia in a high-transmission area of northern Uganda.
Am J Trop Med Hyg.
2013; 88(5): 855–61. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 45.
Skarbinski J, Mwandama D, Wolkon A, et al.:
Impact of indoor residual spraying with lambda-cyhalothrin on malaria parasitemia and anemia prevalence among children less than five years of age in an area of intense, year-round transmission in Malawi.
Am J Trop Med Hyg.
2012; 86(6): 997–1004. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 46.
Temu EA, Coleman M, Abilio AP, et al.:
High prevalence of malaria in Zambezia, Mozambique: The protective effect of IRS versus increased risks due to pig-keeping and house construction.
PLoS One.
2012; 7(2): e31409. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 47.
Bulterys PL, Mharakurwa S, Thuma PE:
Cattle, other domestic animal ownership, and distance between dwelling structures are associated with reduced risk of recurrent Plasmodium falciparum infection in southern Zambia.
Trop Med Int Health.
2009; 14(5): 522–528. PubMed Abstract
| Publisher Full Text
- 48.
Plucinski MM, Chicuecue S, Macete E, et al.:
Evaluation of a universal coverage bed net distribution campaign in four districts in Sofala Province, Mozambique.
Malar J.
2014; 13: 427. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 49.
Singh R, Godson II, Singh S, et al.:
High prevalence of asymptomatic malaria in apparently healthy schoolchildren in Aliero, Kebbi state, Nigeria.
J Vector Borne Dis.
2014; 51(2): 128–132. PubMed Abstract
- 50.
Rulisa S, Kateera F, Bizimana JP, et al.:
Malaria prevalence, spatial clustering and risk factors in a low endemic area of Eastern Rwanda: a cross sectional study.
PLoS One.
2013; 8(7): e69443. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 51.
Matangila JR, Lufuluabu J, Ibalanky AL, et al.:
Asymptomatic Plasmodium falciparum infection is associated with anaemia in pregnancy and can be more cost-effectively detected by rapid diagnostic test than by microscopy in Kinshasa, Democratic Republic of the Congo.
Malar J.
2014; 13: 132. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 52.
Bouyou-Akotet MK, Nzenze-Afene S, Ngoungou EB, et al.:
Burden of malaria during pregnancy at the time of IPTp/SP implementation in Gabon.
Am J Trop Med Hyg.
2010; 82(2): 202–209. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 53.
Rowland M, Webster J, Saleh P, et al.:
Prevention of malaria in Afghanistan through social marketing of insecticide-treated nets: evaluation of coverage and effectiveness by cross-sectional surveys and passive surveillance.
Trop Med Int Health.
2002; 7(10): 813–822. PubMed Abstract
| Publisher Full Text
- 54.
Nankabirwa V, Tylleskar T, Nankunda J, et al.:
Malaria parasitaemia among infants and its association with breastfeeding peer counselling and vitamin A supplementation: a secondary analysis of a cluster randomized trial.
PLoS One.
2011; 6(7): e21862. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 55.
Noor AM, Moloney G, Borle M, et al.:
The use of mosquito nets and the prevalence of Plasmodium falciparum infection in rural South Central Somalia.
PLoS One.
2008; 3(5): e2081. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 56.
Raso G, Silué KD, Vounatsou P, et al.:
Spatial risk profiling of Plasmodium falciparum parasitaemia in a high endemicity area in Côte d’Ivoire.
Malar J.
2009; 8: 252. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 57.
Hii JL, Smith T, Vounatsou P, et al.:
Area effects of bednet use in a malaria-endemic area in Papua New Guinea.
Trans R Soc Trop Med Hyg.
2001; 95(1): 7–13. PubMed Abstract
| Publisher Full Text
- 58.
Littrell M, Sow GD, Ngom A, et al.:
Case investigation and reactive case detection for malaria elimination in northern Senegal.
Malar J.
2013; 12: 331. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 59.
Townes LR, Mwandama D, Mathanga DP, et al.:
Elevated dry-season malaria prevalence associated with fine-scale spatial patterns of environmental risk: a case-control study of children in rural Malawi.
Malar J.
2013; 12: 407. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 60.
Kleinschmidt I, Torrez M, Schwabe C, et al.:
Factors influencing the effectiveness of malaria control in Bioko Island, equatorial Guinea.
Am J Trop Med Hyg.
2007; 76(6): 1027–1032. PubMed Abstract
| Free Full Text
- 61.
Mcclure EM, Meshnick SR, Lazebnik N, et al.:
A cohort study of Plasmodium falciparum malaria in pregnancy and associations with uteroplacental blood flow and fetal anthropometrics in Kenya.
Int J Gynecol Obstet.
2014; 126(1): 78–82. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 62.
Muhindo Mavoko H, Ilombe G, Inocêncio da Luz R, et al.:
Malaria policies versus practices, a reality check from Kinshasa, the capital of the Democratic Republic of Congo.
BMC Public Health.
2015; 15: 352. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 63.
Halliday KE, Karanja P, Turner EL, et al.:
Plasmodium falciparum, anaemia and cognitive and educational performance among school children in an area of moderate malaria transmission: Baseline results of a cluster randomized trial on the coast of Kenya.
Trop Med Int Health.
2012; 17(5): 532–549. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 64.
Baragatti M, Fournet F, Henry MC, et al.:
Social and environmental malaria risk factors in urban areas of Ouagadougou, Burkina Faso.
Malar J.
2009; 8: 13. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 65.
De Beaudrap P, Nabasumba C, Grandesso F, et al.:
Heterogeneous decrease in malaria prevalence in children over a six-year period in south-western Uganda.
Malar J.
2011; 10: 132. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 66.
Osterbauer B, Kapisi J, Bigira V, et al.:
Factors associated with malaria parasitaemia, malnutrition, and anaemia among HIV-exposed and unexposed Ugandan infants: a cross-sectional survey.
Malar J.
2012; 11: 432. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 67.
Satoguina J, Walther B, Drakeley C, et al.:
Comparison of surveillance methods applied to a situation of low malaria prevalence at rural sites in The Gambia and Guinea Bissau.
Malar J.
2009; 8: 274. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 68.
Davis JC, Clark TD, Kemble SK, et al.:
Longitudinal study of urban malaria in a cohort of Ugandan children: description of study site, census and recruitment.
Malar J.
2006; 5: 18. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 69.
Gitonga CW, Edwards T, Karanja PN, et al.:
Plasmodium infection, anaemia and mosquito net use among school children across different settings in Kenya.
Trop Med Int Health.
2012; 17(7): 858–870. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 70.
Bradley J, Matias A, Schwabe C, et al.:
Increased risks of malaria due to limited residual life of insecticide and outdoor biting versus protection by combined use of nets and indoor residual spraying on Bioko Island, Equatorial Guinea.
Malar J.
2012; 11: 242. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 71.
De Beaudrap P, Turyakira E, White LJ, et al.:
Impact of malaria during pregnancy on pregnancy outcomes in a Ugandan prospective cohort with intensive malaria screening and prompt treatment.
Malar J.
2013; 12: 139. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 72.
Hagmann R, Charlwood JD, Gil V, et al.:
Malaria and its possible control on the island of Príncipe.
Malar J.
2003; 2: 15. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 73.
Pullan RL, Bukirwa H, Staedke SG, et al.:
Plasmodium infection and its risk factors in eastern Uganda.
Malar J.
2010; 9: 2. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 74.
Nahum A, Erhart A, Mayé A, et al.:
Malaria incidence and prevalence among children living in a Peri-Urban area on the Coast of Benin, West Africa: A longitudinal study.
Am J Trop Med Hyg.
2010; 83(3): 465–473. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 75.
Sluydts V, Heng S, Coosemans M, et al.:
Spatial clustering and risk factors of malaria infections in Ratanakiri Province, Cambodia.
Malar J.
2014; 13: 387. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 76.
Mmbando BP, Kamugisha ML, Lusingu JP, et al.:
Spatial variation and socio-economic determinants of Plasmodium falciparum infection in northeastern Tanzania.
Malar J.
2011; 10: 145. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 77.
Koram KA, Owusu-Agyei S, Fryauff DJ, et al.:
Seasonal profiles of malaria infection, anaemia, and bednet use among age groups and communities in northern Ghana.
Trop Med Int Health.
2003; 8(9): 793–802. PubMed Abstract
| Publisher Full Text
- 78.
Houngbedji CA, N’Dri PB, Hürlimann E, et al.:
Disparities of Plasmodium falciparum infection, malaria-related morbidity and access to malaria prevention and treatment among school-aged children: a national cross-sectional survey in Côte d’Ivoire.
Malar J.
2015; 14: 7. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 79.
Somi MF, Butler JR, Vahid F, et al.:
Is there evidence for dual causation between malaria and socioeconomic status? Findings from rural Tanzania.
Am J Trop Med Hyg.
2007; 77(6): 1020–1027. PubMed Abstract
- 80.
Ouattara AF, Dagnogo M, Olliaro PL, et al.:
Plasmodium falciparum infection and clinical indicators in relation to net coverage in central Côte d’Ivoire.
Parasit Vectors.
2014; 7: 306. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 81.
Sarpong N, Owusu-Dabo E, Kreuels B, et al.:
Prevalence of malaria parasitaemia in school children from two districts of Ghana earmarked for indoor residual spraying: a cross-sectional study.
Malar J.
2015; 14: 260. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 82.
Hamer DH, Singh MP, Wylie BJ, et al.:
Burden of malaria in pregnancy in Jharkhand State, India.
Malar J.
2009; 8: 210. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 83.
Mwangi TW, Ross A, Marsh K, et al.:
The effects of untreated bednets on malaria infection and morbidity on the Kenyan coast.
Trans R Soc Trop Med Hyg.
2003; 97(4): 369–372. PubMed Abstract
| Publisher Full Text
- 84.
Trape JF, Tall A, Diagne N, et al.:
Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study.
Lancet Infect Dis.
2011; 11(12): 925–932. PubMed Abstract
| Publisher Full Text
- 85.
Wotodjo AN, Diagne N, Gaudart J, et al.:
Malaria risk factors in Dielmo, a Senegalese malaria-endemic village, between October and November of 2013: a case-control study.
Am J Trop Med Hyg.
2015; 92(3): 565–8. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 86.
Rogier C, Henry MC, Luxemburger C:
Methods for the phase IV evaluation of malaria vector control interventions: a case-control study of the effectiveness of long lasting impregnated bed nets after their deployment in Benin.
Med Trop (Mars).
2009; 69(2): 195–202. PubMed Abstract
- 87.
Wotodjo AN, Richard V, Boyer S, et al.:
The implication of long-lasting insecticide-treated net use in the resurgence of malaria morbidity in a Senegal malaria endemic village in 2010-2011.
Parasit Vectors.
2015; 8: 267. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 88.
Nonaka D, Maazou A, Yamagata S, et al.:
Can Long-lasting Insecticide-treated Bednets with Holes Protect Children from Malaria?
Trop Med Health.
2014; 42(3): 99–105. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 89.
Okebe J, Mwesigwa J, Kama EL, et al.:
A comparative case control study of the determinants of clinical malaria in The Gambia.
Malar J.
2014; 13: 306. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 90.
Moiroux N, Boussari O, Djènontin A, et al.:
Dry season determinants of malaria disease and net use in Benin, West Africa.
PLoS One.
2012; 7(1): e30558. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 91.
Shirayama Y, Phompida S, Kuroiwa C, et al.:
Maintenance behaviour and long-lasting insecticide-treated nets (LLITNs) previously introduced into Bourapar district, Khammouane province, Lao PDR.
Public Health.
2007; 121(2): 122–129. PubMed Abstract
| Publisher Full Text
- 92.
Rowland M, Hewitt S, Durrani N, et al.:
Sustainability of pyrethroid-impregnated bednets for malaria control in Afghan communities.
Bull World Health Organ.
1997; 75(1): 23–29. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 93.
Ugwu EO, Ezechukwu PC, Obi SN, et al.:
Utilization of insecticide treated nets among pregnant women in Enugu, South Eastern Nigeria.
Niger J Clin Pract.
2013; 16(3): 292–296. PubMed Abstract
| Publisher Full Text
- 94.
Henry MC, Doannio JM, Darriet F, et al.:
Efficacy of permethrin-impregnated Olyset Net mosquito nets in a zone with pyrethroid resistance vectors. II. Parasitic and clinical evaluation.
Med Trop (Mars).
1999; 59(4): 355–7. PubMed Abstract
- 95.
Bejon P, Ogada E, Peshu N, et al.:
Interactions between age and ITN use determine the risk of febrile malaria in children.
PLoS One.
2009; 4(12): e8321. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 96.
Alexander N, Rodríguez M, Pérez L, et al.:
Case-Control study of mosquito nets against malaria in the Amazon region of Colombia.
Am J Trop Med Hyg.
2005; 73(1): 140–148. PubMed Abstract
- 97.
Mathanga DP, Campbell CH, Taylor TE, et al.:
Reduction of childhood malaria by social marketing of insecticide-treated nets: a case-control study of effectiveness in Malawi.
Am J Trop Med Hyg.
2005; 73(3): 622–625. PubMed Abstract
- 98.
Clark TD, Greenhouse B, Njama-Meya D, et al.:
Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda.
J Infect Dis.
2008; 198(3): 393–400. PubMed Abstract
| Publisher Full Text
- 99.
Kamya MR, Gasasira AF, Achan J, et al.:
Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children.
AIDS.
2007; 21(15): 2059–66. PubMed Abstract
| Publisher Full Text
- 100.
Oladeinde B, Omoregie R, Olley M, et al.:
Malaria and Anemia among Children in a Low Resource Setting In Nigeria.
Iran J Parasitol.
2012; 7(3): 31–37. PubMed Abstract
| Free Full Text
- 101.
Loha E, Lindtjørn B:
Predictors of Plasmodium falciparum Malaria Incidence in Chano Mille, South Ethiopia: a longitudinal study.
Am J Trop Med Hyg.
2012; 87(3): 450–459. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 102.
Lindblade KA, Mwandama D, Mzilahowa T, et al.:
A cohort study of the effectiveness of insecticide-treated bed nets to prevent malaria in an area of moderate pyrethroid resistance, Malawi.
Malar J.
2015; 14: 31. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 103.
Dickinson KL, Randell HF, Kramer RA, et al.:
Socio-economic status and malaria-related outcomes in Mvomero District, Tanzania.
Glob Public Health.
2012; 7(4): 384–399. PubMed Abstract
| Publisher Full Text
- 104.
Abdella YM, Deribew A, Kassahun W:
Does Insecticide Treated Mosquito Nets (ITNs) prevent clinical malaria in children aged between 6 and 59 months under program setting?
J Community Health.
2009; 34(2): 102–12. PubMed Abstract
| Publisher Full Text
- 105.
Macedo De Oliveira A, Mutemba R, Morgan J, et al.:
Prevalence of malaria among patients attending public health facilities in Maputo City, Mozambique.
Am J Trop Med Hyg.
2011; 85(6): 1002–1007. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 106.
Yamamoto SS, Louis VR, Sié A, et al.:
The effects of zooprophylaxis and other mosquito control measures against malaria in Nouna, Burkina Faso.
Malar J.
2009; 8: 283. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 107.
Roosihermiatie B, Nishiyama M, Nakae K:
The human behavioral and socioeconomic determinants of malaria in Bacan Island, North Maluku, Indonesia.
J Epidemiol.
2000; 10(4): 280–289. PubMed Abstract
| Publisher Full Text
- 108.
Sharma PK, Ramanchandran R, Hutin YJ, et al.:
A malaria outbreak in Naxalbari, Darjeeling district, West Bengal, India, 2005: weaknesses in disease control, important risk factors.
Malar J.
2009; 8: 288. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 109.
Deressa W, Ali A, Berhane Y:
Household and socioeconomic factors associated with childhood febrile illnesses and treatment seeking behaviour in an area of epidemic malaria in rural Ethiopia.
Trans R Soc Trop Med Hyg.
2007; 101(9): 939–947. PubMed Abstract
| Publisher Full Text
- 110.
Haque U, Glass GE, Bomblies A, et al.:
Risk factors associated with clinical malaria episodes in Bangladesh: a longitudinal study.
Am J Trop Med Hyg.
2013; 88(4): 727–732. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 111.
Ong’echa JM, Keller CC, Were T, et al.:
Parasitemia, anemia, and malarial anemia in infants and young children in a rural holoendemic Plasmodium falciparum transmission area.
Am J Trop Med Hyg.
2006; 74(3): 376–385. PubMed Abstract
- 112.
Byakika-Kibwika P, Ndeezi G, Kamya MR:
Health care related factors associated with severe malaria in children in Kampala, Uganda.
Afr Health Sci.
2009; 9(3): 206–210. PubMed Abstract
| Free Full Text
- 113.
Njama D, Dorsey G, Guwatudde D, et al.:
Urban malaria: Primary caregivers’ knowledge, attitudes, practices and predictors of malaria incidence in a cohort of Ugandan children.
Trop Med Int Health.
2003; 8(8): 685–692. PubMed Abstract
| Publisher Full Text
- 114.
Webster J, Chandramohan D, Freeman T, et al.:
A health facility based case-control study of effectiveness of insecticide treated nets: potential for selection bias due to pre-treatment with chloroquine.
Trop Med Int Health.
2003; 8(3): 196–201. PubMed Abstract
| Publisher Full Text
- 115.
Snow RW, Peshu N, Forster D, et al.:
Environmental and entomological risk factors for the development of clinical malaria among children on the Kenyan coast.
Trans R Soc Trop Med Hyg.
1998; 92(4): 381–385. PubMed Abstract
| Publisher Full Text
- 116.
Ernst KC, Lindblade KA, Koech D, et al.:
Environmental, socio-demographic and behavioural determinants of malaria risk in the western Kenyan highlands: a case-control study.
Trop Med Int Health.
2009; 14(10): 1258–1265. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 117.
Coleman M, Coleman M, Mabaso ML, et al.:
Household and microeconomic factors associated with malaria in Mpumalanga, South Africa.
Trans R Soc Trop Med Hyg.
2010; 104(2): 143–147. PubMed Abstract
| Publisher Full Text
- 118.
Yusuf OB, Adeoye BW, Oladepo OO, et al.:
Poverty and fever vulnerability in Nigeria: a multilevel analysis.
Malar J.
2010; 9: 235. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 119.
Siri JG, Wilson ML, Murray S, et al.:
Significance of travel to rural areas as a risk factor for malarial anemia in an urban setting.
Am J Trop Med Hyg.
2010; 82(3): 391–397. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 120.
Carme B, Plassart H, Senga P, et al.:
Cerebral malaria in African children: socioeconomic risk factors in Brazzaville, Congo.
Am J Trop Med Hyg.
1994; 50(2): 131–136. PubMed Abstract
| Publisher Full Text
- 121.
Guthmann JP, Hall AJ, Jaffar S, et al.:
Environmental risk factors for clinical malaria: a case-control study in the Grau region of Peru.
Trans R Soc Trop Med Hyg.
2001; 95(6): 577–583. PubMed Abstract
| Publisher Full Text
- 122.
Komazawa O, Kaneko S, K’Opiyo J, et al.:
Are long-lasting insecticidal nets effective for preventing childhood deaths among non-net users? A community-based cohort study in western Kenya.
PLoS One.
2012; 7(11): e49604. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 123.
Fegan GW, Noor AM, Akhwale WS, et al.:
Effect of expanded insecticide-treated bednet coverage on child survival in rural Kenya: a longitudinal study.
Lancet.
2007; 370(9592): 1035–9. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 124.
Schellenberg JR, Abdulla S, Nathan R, et al.:
Effect of large-scale social marketing of Insecticide-treated nets on child survival in rural Tanzania.
Lancet.
2001; 357(9264): 1241–1247. PubMed Abstract
| Publisher Full Text
- 125.
Eisele TP, Larsen DA, Anglewicz PA, et al.:
Malaria prevention in pregnancy, birthweight, and neonatal mortality: a meta-analysis of 32 national cross-sectional datasets in Africa.
Lancet Infect Dis.
2012; 12(12): 942–949. PubMed Abstract
| Publisher Full Text
- 126.
Smith T, Genton B, Betuela I, et al.:
Mosquito nets for the elderly?
Trans R Soc Trop Med Hyg.
2002; 96(1): 37–38. PubMed Abstract
| Publisher Full Text
- 127.
Gosoniu L, Vounatsou P, Tami A, et al.:
Spatial effects of mosquito bednets on child mortality.
BMC Public Health.
2008; 8: 356. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 128.
Kabanywanyi AM, Macarthur JR, Stolk WA, et al.:
Malaria in pregnant women in an area with sustained high coverage of insecticide-treated bed nets.
Malar J.
2008; 7: 133. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 129.
Ezebialu IU, Eke AC, Ezeagwuna DA, et al.:
Prevalence, pattern, and determinants of placental malaria in a population of southeastern Nigerian parturients.
Int J Infect Dis.
2012; 16(12): e860–5. PubMed Abstract
| Publisher Full Text
- 130.
Vanga-Bosson HA, Coffie PA, Kanhon S, et al.:
Coverage of intermittent prevention treatment with sulphadoxine-pyrimethamine among pregnant women and congenital malaria in Côte d’Ivoire.
Malar J.
2011; 10: 105. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 131.
Gutman J, Mwandama D, Wiegand RE, et al.:
Effectiveness of intermittent preventive treatment with sulfadoxine-pyrimethamine during pregnancy on maternal and birth outcomes in Machinga district, Malawi.
J Infect Dis.
2013; 208(6): 907–916. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 132.
Nganda RY, Drakeley C, Reyburn H, et al.:
Knowledge of malaria influences the use of insecticide treated nets but not intermittent presumptive treatment by pregnant women in Tanzania.
Malar J.
2004; 3: 42. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 133.
Msyamboza K, Senga E, Tetteh-Ashong E, et al.:
Estimation of effectiveness of interventions for malaria control in pregnancy using the screening method.
Int J Epidemiol.
2007; 36(2): 406–411. PubMed Abstract
| Publisher Full Text
- 134.
Tongo OO, Orimadegun AE, Akinyinka OO:
Utilisation of malaria preventive measures during pregnancy and birth outcomes in Ibadan, Nigeria.
BMC Pregnancy Childbirth.
2011; 11: 60. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 135.
Mpogoro FJ, Matovelo D, Dosani A, et al.:
Uptake of intermittent preventive treatment with sulphadoxine-pyrimethamine for malaria during pregnancy and pregnancy outcomes: a cross-sectional study in Geita district, North-Western Tanzania.
Malar J.
2014; 13: 455. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 136.
Namusoke F, Rasti N, Kironde F, et al.:
Malaria burden in pregnancy at mulago national referral hospital in kampala, Uganda.
Malar Res Treat.
2010; 2010: 913857. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 137.
Tonga C, Kimbi HK, Anchang-Kimbi JK, et al.:
Malaria risk factors in women on intermittent preventive treatment at delivery and their effects on pregnancy outcome in Sanaga-Maritime, Cameroon.
PLoS One.
2013; 8(8): e65876. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 138.
Kurth F, Bélard S, Mombo-Ngoma G, et al.:
Adolescence as risk factor for adverse pregnancy outcome in Central Africa--a cross-sectional study.
PLoS One.
2010; 5(12): e14367. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 139.
Sintasath DM, Ghebremeskel T, Lynch M, et al.:
Malaria prevalence and associated risk factors in Eritrea.
Am J Trop Med Hyg.
2005; 72(6): 682–687. PubMed Abstract
- 140.
Mauny F, Viel JF, Handschumacher P, et al.:
Multilevel modelling and malaria: a new method for an old disease.
Int J Epidemiol.
2004; 33(6): 1337–1344. PubMed Abstract
| Publisher Full Text
- 141.
Gies S, Coulibaly SO, Ouattara FT, et al.:
Individual efficacy of intermittent preventive treatment with sulfadoxine-pyrimethamine in primi- and secundigravidae in rural Burkina Faso: impact on parasitaemia, anaemia and birth weight.
Trop Med Int Health.
2009; 14(2): 174–82. PubMed Abstract
| Publisher Full Text
- 142.
van Eijk AM, Ayisi JG, ter Kuile FO, et al.:
Effectiveness of intermittent preventive treatment with sulphadoxine-pyrimethamine for control of malaria in pregnancy in western Kenya: a hospital-based study.
Trop Med Int Health.
2004; 9(3): 351–360. PubMed Abstract
| Publisher Full Text
- 143.
Mbonye AK, Bygbjerg I, Magnussen P:
Intermittent preventive treatment of malaria in pregnancy: a community-based delivery system and its effect on parasitemia, anemia and low birth weight in Uganda.
Int J Infect Dis.
2008; 12(1): 22–29. PubMed Abstract
| Publisher Full Text
- 144.
Rogawski ET, Chaluluka E, Molyneux ME, et al.:
The effects of malaria and intermittent preventive treatment during pregnancy on fetal anemia in Malawi.
Clin Infect Dis.
2012; 55(8): 1096–1102. PubMed Abstract
| Publisher Full Text
- 145.
Ramharter M, Schuster K, Bouyou-Akotet MK, et al.:
Malaria in pregnancy before and after the implementation of a national IPTp program in Gabon.
Am J Trop Med Hyg.
2007; 77(3): 418–422. PubMed Abstract
- 146.
Toure OA, Kone PL, Coulibaly MA, et al.:
Coverage and efficacy of intermittent preventive treatment with sulphadoxine pyrimethamine against malaria in pregnancy in Côte d’Ivoire five years after its implementation.
Parasit Vectors.
2014; 7: 495. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 147.
Wilson NO, Ceesay FK, Obed SA, et al.:
Intermittent preventive treatment with sulfadoxine-pyrimethamine against malaria and anemia in pregnant women.
Am J Trop Med Hyg.
2011; 85(1): 12–21. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 148.
Mosha D, Chilongola J, Ndeserua R, et al.:
Effectiveness of intermittent preventive treatment with sulfadoxine-pyrimethamine during pregnancy on placental malaria, maternal anaemia and birthweight in areas with high and low malaria transmission intensity in Tanzania.
Trop Med Int Health.
2014; 19(9): 1048–56. PubMed Abstract
| Publisher Full Text
- 149.
Bako BG, Audu BM, Geidam AD, et al.:
Prevalence, risk factors and effects of placental malaria in the UMTH, Maiduguri, North-eastern, Nigeria: a cross-sectional study.
J Obstet Gynaecol.
2009; 29(4): 307–310. PubMed Abstract
| Publisher Full Text
- 150.
Hommerich L, von Oertzen C, Bedu-Addo G, et al.:
Decline of placental malaria in southern Ghana after the implementation of intermittent preventive treatment in pregnancy.
Malar J.
2007; 6: 144. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 151.
Sirima SB, Cotte AH, Konaté A, et al.:
Malaria prevention during pregnancy: assessing the disease burden one year after implementing a program of intermittent preventive treatment in Koupela District, Burkina Faso.
Am J Trop Med Hyg.
2006; 75(2): 205–211. PubMed Abstract
- 152.
Peter AO:
Effect of intermittent preventive treatment of malaria on the outcome of pregnancy among women attending antenatal clinic of a new Nigerian teaching hospital, Ado-Ekiti.
Niger Med J.
2013; 54(3): 170–5. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 153.
Falade CO, Yusuf BO, Fadero FF, et al.:
Intermittent preventive treatment with sulphadoxine-pyrimethamine is effective in preventing maternal and placental malaria in Ibadan, south-western Nigeria.
Malar J.
2007; 6: 88. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 154.
Harrington WE, Mutabingwa TK, Kabyemela E, et al.:
Intermittent treatment to prevent pregnancy malaria does not confer benefit in an area of widespread drug resistance.
Clin Infect Dis.
2011; 53(3): 224–230. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 155.
Famanta A, Diakite M, Diawara SI, et al.:
[Prevalence of maternal and placental malaria and of neonatal low birth weight in a semi-urban area of Bamako (Mali)].
Sante.
2011; 21(1): 3–7. PubMed Abstract
| Publisher Full Text
- 156.
Harrington WE, Morrison R, Fried M, et al.:
Intermittent preventive treatment in pregnant women is associated with increased risk of severe malaria in their offspring.
PLoS One.
2013; 8(2): e56183. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 157.
Anchang-Kimbi JK, Achidi EA, Nkegoum B, et al.:
Diagnostic comparison of malaria infection in peripheral blood, placental blood and placental biopsies in Cameroonian parturient women.
Malar J.
2009; 8: 126. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 158.
Mace KE, Chalwe V, Katalenich BL, et al.:
Evaluation of sulphadoxine-pyrimethamine for intermittent preventive treatment of malaria in pregnancy: a retrospective birth outcomes study in Mansa, Zambia.
Malar J.
2015; 14: 69. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 159.
Valea I, Tinto H, Drabo MK, et al.:
An analysis of timing and frequency of malaria infection during pregnancy in relation to the risk of low birth weight, anaemia and perinatal mortality in Burkina Faso.
Malar J.
2012; 11: 71. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 160.
Arinaitwe E, Ades V, Walakira A, et al.:
Intermittent preventive therapy with sulfadoxine-pyrimethamine for malaria in pregnancy: a cross-sectional study from Tororo, Uganda.
PLoS One.
2013; 8(9): e73073. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 161.
Likwela JL, D’Alessandro U, Lokwa BL, et al.:
Sulfadoxine-pyrimethamine resistance and intermittent preventive treatment during pregnancy: a retrospective analysis of birth weight data in the Democratic Republic of Congo (DRC).
Trop Med Int Health.
2012; 17(3): 322–329. PubMed Abstract
| Publisher Full Text
- 162.
Apinjoh TO, Anchang-Kimbi JK, Mugri RN, et al.:
Determinants of infant susceptibility to malaria during the first year of life in South Western cameroon.
Open Forum Infect Dis.
2015; 2(1): ofv012. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 163.
Al-Taiar A, Assabri A, Al-Habori M, et al.:
Socioeconomic and environmental factors important for acquiring non-severe malaria in children in Yemen: a case-control study.
Trans R Soc Trop Med Hyg.
2009; 103(1): 72–78. PubMed Abstract
| Publisher Full Text
- 164.
Srinivas G, Edwin Amalraj R, Dhanraj B:
The use of personal protection measures against malaria in an urban population.
Public Health.
2005; 119(5): 415–417. PubMed Abstract
| Publisher Full Text
- 165.
Willey BA, Armstrong Schellenberg JR, Maokola W, et al.:
Evaluating the effectiveness of IPTi on malaria using routine health information from sentinel health centres in southern Tanzania.
Malar J.
2011; 10: 41. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 166.
Bowen HL:
Impact of a mass media campaign on bed net use in Cameroon.
Malar J.
2013; 12: 36. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 167.
Boulay M, Lynch M, Koenker H:
Comparing two approaches for estimating the causal effect of behaviour-change communication messages promoting insecticide-treated bed nets: an analysis of the 2010 Zambia malaria indicator survey.
Malar J.
2014; 13: 342. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 168.
Lengeler C:
Insecticide-treated bed nets and curtains for preventing malaria.
Cochrane Database Syst Rev.
2004; (2): CD000363. PubMed Abstract
| Publisher Full Text
- 169.
Pluess B, Tanser FC, Lengeler C, et al.:
Indoor residual spraying for preventing malaria.
Cochrane Database Syst Rev.
2010; (4): CD006657. PubMed Abstract
| Publisher Full Text
- 170.
Kesteman T, Randrianarivelojosia M, Mattern C, et al.:
Nationwide evaluation of malaria infections, morbidity, mortality, and coverage of malaria control interventions in Madagascar.
Malar J.
2014; 13: 465. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 171.
Gamble C, Ekwaru JP, ter Kuile FO:
Insecticide-treated nets for preventing malaria in pregnancy.
Cochrane Database Syst Rev.
2009; (2): CD003755. PubMed Abstract
| Publisher Full Text
- 172.
Kim D, Fedak K, Kramer R:
Reduction of malaria prevalence by indoor residual spraying: a meta-regression analysis.
Am J Trop Med Hyg.
2012; 87(1): 117–24. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 173.
Eisele TP, Larsen D, Steketee RW:
Protective efficacy of interventions for preventing malaria mortality in children in Plasmodium falciparum endemic areas.
Int J Epidemiol.
2010; 39 Suppl 1: i88–101. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 174.
Guyatt HL, Corlett SK, Robinson TP, et al.:
Malaria prevention in highland Kenya: indoor residual house-spraying vs. insecticide-treated bednets.
Trop Med Int Health.
2002; 7(4): 298–303. PubMed Abstract
| Publisher Full Text
- 175.
Rowland M, Bouma M, Ducornez D, et al.:
Pyrethroid-impregnated bed nets for personal protection against malaria for Afghan refugees.
Trans R Soc Trop Med Hyg.
1996; 90(4): 357–361. PubMed Abstract
| Publisher Full Text
- 176.
Misra SP, Webber R, Lines J, et al.:
Malaria control: bednets or spraying? Spray versus treated nets using deltamethrin--a community randomized trial in India.
Trans R Soc Trop Med Hyg.
1999; 93(5): 456–457. PubMed Abstract
| Publisher Full Text
- 177.
Molineaux L, Gramiccia G: The Garki Project. Research on the Epidemiology and Control of Malaria in the Sudan Savanna of West Africa. Geneva; 1980. Reference Source
- 178.
Curtis CE, Maxwell CA, Finch RJ, et al.:
A comparison of use of a pyrethroid either for house spraying or for bednet treatment against malaria vectors.
Trop Med Int Health.
1998; 3(8): 619–631. PubMed Abstract
| Publisher Full Text
- 179.
Hamel MJ, Otieno P, Bayoh N, et al.:
The combination of indoor residual spraying and insecticide-treated nets provides added protection against malaria compared with insecticide-treated nets alone.
Am J Trop Med Hyg.
2011; 85(6): 1080–6. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 180.
West PA, Protopopoff N, Wright A, et al.:
Indoor residual spraying in combination with insecticide-treated nets compared to insecticide-treated nets alone for protection against malaria: a cluster randomised trial in Tanzania.
PLoS Med.
2014; 11(4): e1001630. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 181.
Corbel V, Akogbeto M, Damien GB, et al.:
Combination of malaria vector control interventions in pyrethroid resistance area in Benin: a cluster randomised controlled trial.
Lancet Infect Dis.
2012; 12(8): 617–26. PubMed Abstract
| Publisher Full Text
- 182.
Kafy H:
Combination of IRS with LLINs versus LLINS alone in Sudan: results of a very large randomised trial. In 6th MIM Pan-African Malar Conf. Durban, South Africa; 2013.
- 183.
Pinder M, Jawara M, Jarju LB, et al.:
Efficacy of indoor residual spraying with dichlorodiphenyltrichloroethane against malaria in Gambian communities with high usage of long-lasting insecticidal mosquito nets: a cluster-randomised controlled trial.
Lancet.
2015; 385(9976): 1436–1446. PubMed Abstract
| Publisher Full Text
- 184.
Vector Control Technical Expert Group: WHO Guidance for Countries on Combining Indoor Residual Spraying and Long-Lasting Insecticidal Nets. Geneva; 2014. Reference Source
- 185.
ter Kuile FO, van Eijk AM, Filler SJ:
Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review.
JAMA.
2007; 297(23): 2603–2616. PubMed Abstract
| Publisher Full Text
- 186.
Mbaye A, Richardson K, Balajo B, et al.:
A randomized, placebo-controlled trial of intermittent preventive treatment with sulphadoxine-pyrimethamine in Gambian multigravidae.
Trop Med Int Health.
2006; 11(7): 992–1002. PubMed Abstract
| Publisher Full Text
- 187.
Ishaque S, Yakoob MY, Imdad A, et al.:
Effectiveness of interventions to screen and manage infections during pregnancy on reducing stillbirths: a review.
BMC Public Health.
2011; 11 Suppl 3: S3. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 188.
Naugle DA, Hornik RC:
Systematic review of the effectiveness of mass media interventions for child survival in low- and middle-income countries.
J Health Commun.
2014; 19(Suppl 1): 190–215. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 189.
Kesteman T, Rogier C, Milijaona R:
Dataset 1 in: The protective effectiveness of control interventions for malaria prevention: a systematic review of the literature.
F1000Research.
2017. Data Source




Comments on this article Comments (0)