Keywords
Predator outbreak, Acanthaster, Mutualistic defense, Guardian crab, Trapezia, Mixed-species predator guild, Trophic cascade, Density dependence.
This article is included in the Ecology and Global Change gateway.
Predator outbreak, Acanthaster, Mutualistic defense, Guardian crab, Trapezia, Mixed-species predator guild, Trophic cascade, Density dependence.
We have updated our manuscript following judicious comments provided by the referees. The new version notably includes further information, as well as an extended discussion, on the reported ecological process and its prevalence in our study system. The new version also provides more details on coral mutualist crustaceans and clearer Figures 1, 2, and Supplementary Image 1. Following comments from the referees, we have added arrows to the figures, in order to clarify which parts we are referring to. We are thankful to the referees for their constructive remarks.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Identifying ecological processes that drive species trajectories is a prerequisite for ecosystem management. However, community dynamics are sometimes governed by unexpected, indirect interactions and complex emergent properties that can cause runaway responses and abrupt ecological shifts (Silliman et al., 2013; Terborgh & Estes, 2010). Outbreaks of the coral predator crown-of-thorns seastar (COTS) cause widespread coral mortality across the Indo-Pacific Ocean (Pratchett et al., 2014) with often drastic impacts on diverse reef communities (Kayal et al., 2012). However, some coral species possess mutualistic allies that can deter COTS predation. In particular, trapeziid crabs and alpheid shrimps inhabiting large pocilloporids are known for their ability to effectively defend their host corals from COTS assaults (Glynn, 2013; McKeon & Moore, 2014), although guarded pocilloporids do not always survive COTS outbreaks (Leray et al., 2012; see Figure 1). Despite increasing understanding of factors determining coral susceptibility to COTS predation (Glynn, 1976; Kayal et al., 2011; Kayal & Kayal, 2017; Pratchett, 2001; Rouzé et al., 2014), the processes sealing the fate of guarded corals during outbreaks have remained unknown. Here we provide insights into the ecological mechanisms underlying the fall of guarded corals during predatory COTS outbreaks.
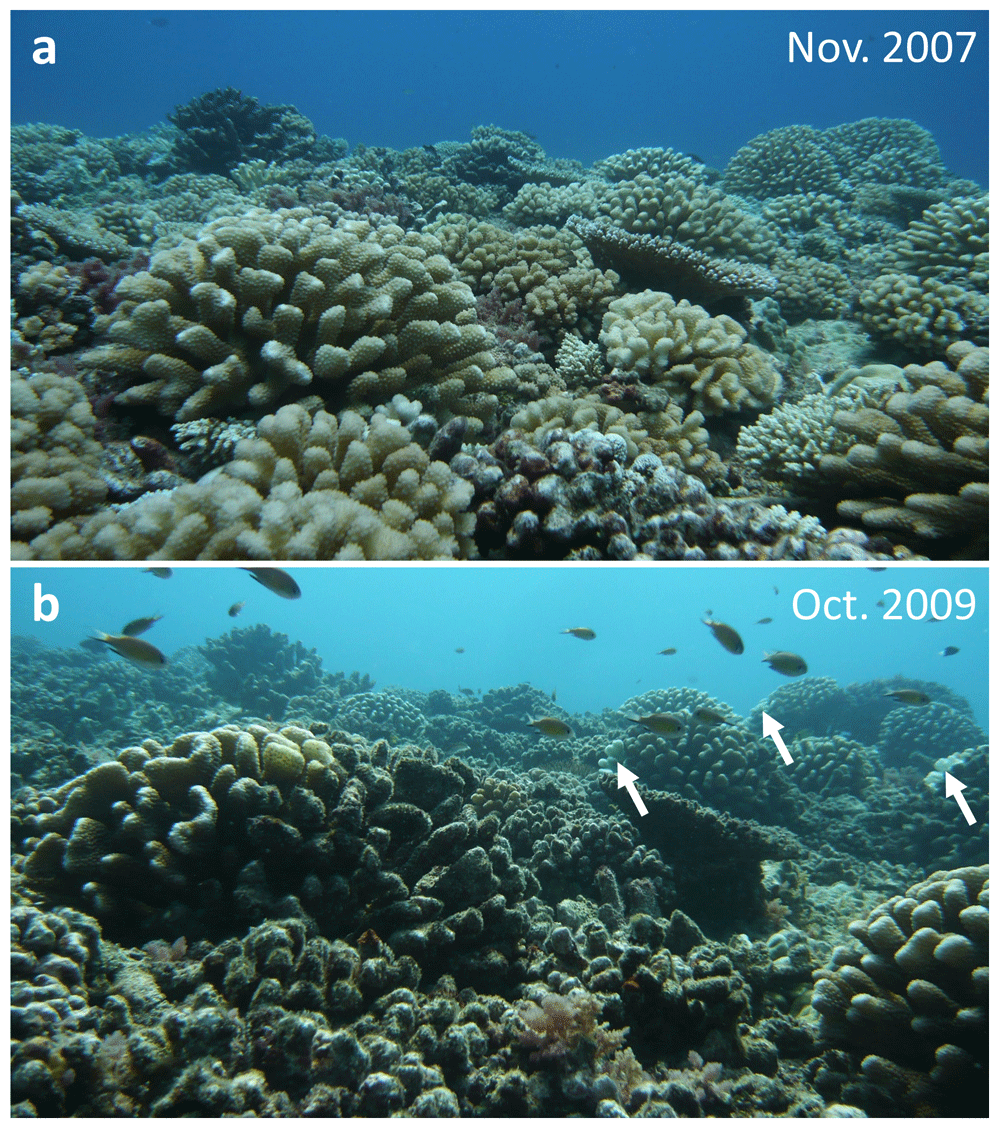
Pictures were taken at 6 m depth on Tiahura reef in Moorea, French Polynesia, before (a) and after (b) this location was invaded by crown-of-thorns seastar (COTS) swarms. Surviving guarded corals typically measured above 60 cm in diameter. Arrows in b indicate white feeding scars characteristic of recent COTS predation on several of the guarded coral colonies (Pocillopora eydouxi).
Our observations were performed at the peak of an intense crown-of-thorns seastar (COTS) outbreak that decimated coral communities around the island of Moorea (17.539° S, 149.830° W), French Polynesia, between 2003 and 2010. General patterns in propagation of COTS swarms around the island, and impacts on corals and other reef communities were described by Kayal et al. (2012); Kayal et al. (2017). Here, we provide complementary observations that unveil processes leading to the fall of large (typically above 60 cm diameter) pocilloporid assemblages that benefit from “anti-COTS” mutualistic defense, the so-called guarded corals. In Moorea, these assemblages are dominated by Pocillopora eydouxi, a species that hosts trapeziid crabs and alpheid shrimps able to deter COTS predation (Glynn, 2013; Leray et al., 2012; McKeon & Moore, 2014; Figure 1). Our observations were performed using SCUBA on the outer reef slope at Tiahura where the COTS outbreaks in Moorea were initiated and had particularly detrimental impacts (Kayal et al., 2012).
In August 2008 at 12 m depth on Tiahura reef, we observed an unusually dense aggregation of coral-eating butterflyfishes jamming around guarded pocilloporids, the last coral bastions that had yet resisted swarms of the predatory seastar (Figure 2, Supplementary Image 1). Widespread coral decline had previously wiped out much of resident populations of coral-feeding butterflyfishes (Kayal et al., 2012), pushing starving survivors to aggregate around the guarded corals. While butterflyfishes were increasingly observed to gather around guarded corals as the COTS outbreak progressed around the island, the aggregation of 9 butterflyfishes within a single square-meter (9 fish.m-2), as captured in Figure 2, was particularly surprising. Density of the coral-feeding butterflyfish assemblage on this reef location had dropped to the much lower average value of 4.3±0.9 SE fish.200m-2 following the COTS outbreak (surveyed in June 2008, equivalent to 0.02 fish.m-2). The observed aggregation thus represented a more than 400-times concentration of the predation pressure exerted by the butterflyfishes, and was targeting a guarded pocilloporid that was already under attack by COTS (Figure 2, Supplementary Image 1).

This aggregation was observed following widespread coral decline (note the absence of live coral in the background) in August 2008 at 12 m depth on Tiahura reef in Moorea, French Polynesia. The guarded coral measured approximately 70 cm in diameter. The predator guild was composed of a crown-of-thorns seastar (COTS) and nine butterflyfishes from species Chaetodon ornatissimus, C. pelewensis, C. quadrimaculatus, C. reticulatus. Arrows indicate white feeding scars characteristic of recent COTS predation on the guarded coral (Pocillopora eydouxi).
Guarded pocilloporids in Moorea have shown the ability to resist devastating COTS predation for several years (McKeon & Moore, 2014; Figure 1). However, concurrent assaults from such locally amplified, mixed-species predatory guilds likely overwhelm the ability of trapeziid crabs and other exo-symbionts to defend host pocilloporids, ultimately causing the fall of guarded corals. Indeed, coral occupation by mutualist communities is determined by strict rules of territoriality and competition (Glynn, 2013; Leray et al., 2012), which limits the abundance of inhabiting guardians in host colonies, and therefore their ability to sustain predatory assaults. The relative contribution of the butterflyfishes, as compared to COTS, to the death of guarded corals at this stage remains unclear. Further research is needed to quantitatively evaluate the aptitude of coral mutualists to withstand attacks from mono- versus multi-specific predators at different abundances. This particularly applies when the predator guilds involve specialized coral-feeding species from distant phyla with different feeding modes, such as fishes that sample polyps through repeated rapid bites and seastars that consume large portions of coral tissue over extended amount of time. Coral decline has already been identified as an engine of COTS movements and prey selection during outbreaks (Kayal et al., 2011; Kayal et al., 2012; Silliman et al., 2013). Our observations suggest that further cascading effects include aggregating diverse predators in numbers surpassing mutualistic defenses, eventually leading to the collapse of guarded corals. We therefore advocate the importance of controlling COTS outbreaks at the earliest stages, before trophic cascades could lead to a runaway collapse of coral communities.
All data underlying the results are available as part of the article and no additional source data are required.
Mohsen Kayal’s Ph.D. studies were supported by grants from Polynésienne des Eaux and Planète Urgence.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Image 1. Additional photographs capturing the predatory guild aggregating around a guarded coral following widespread coral decline. Pictures (a, b) were taken in August 2008 at 12 m depth on Tiahura reef in Moorea, French Polynesia, at the peak of an intense outbreak of the coral-eating crown-of-thorns seastar (COTS). The observed macro-predator aggregation was composed of one COTS and nine individuals from resident butterflyfish species Chaetodon ornatissimus, C. pelewensis, C. quadrimaculatus, C. reticulatus (see Figure 2). White feeding scars characteristic of recent COTS predation can be seen on the guarded coral (Pocillopora eydouxi).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Reef coral biology and ecology.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 02 Mar 18 |
||
|
Version 1 13 Nov 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)