Keywords
axonal transport, intravital imaging, neurons
axonal transport, intravital imaging, neurons
Neurons are highly polarised, excitable cells with long, thin axons whose integrity requires specialised mechanisms to transport cargoes such as organelles (e.g. mitochondria) and molecules (e.g. proteins and RNA) in anterograde (from soma to axonal tips) and retrograde (from axonal tips to soma) directions1. This bi-directional axonal transport is governed by the kinesin and cytoplasmic dynein motor proteins, respectively, and is essential for neuronal survival and function2. Given the large distances over which these processes must occur, it is perhaps unsurprising that disturbances in axonal transport have been linked to both ageing and many severe nervous system diseases, including Alzheimer’s disease (AD) and amyotrophic lateral sclerosis (ALS)3–5. Emphasising the importance of efficient axonal transport to nervous system health, mutations in a number of motor proteins have been identified as causative in neuronal disorders1,6,7.
Individual cargoes have long been tracked in real-time in primary neuron and ex vivo tissue axons8–10; however, there is evidence to suggest that these artificial environments do not consistently reflect the in vivo situation11–14. Differences in transport dynamics, such as average speeds and pause frequencies11, could be attributed to limitations inherent to these in vitro and ex vivo platforms. Cultured primary neurons lack the array of cells with which neurons normally physically and chemically interact in situ; for example, cultured motor neurons are not myelinated, do not contact target muscle cells, and lack a network of regulated excitatory and inhibitory input onto their cell bodies. Myelination15–17, target-derived signals18,19, and activity20–23 are all known to impact axonal transport, whereas non-cell autonomous and age-dependent disease mechanisms are difficult to accurately model in vitro. Mouse primary cultures are often derived from embryonic animals24,25 not currently analysed in vivo, which may also cause discrepancies, as could the current intrinsic variability of human induced pluripotent stem cell (hiPSC)-derived neuronal cultures26. Furthermore, the artificially controlled in vitro environment could affect subcellular energy demands and transport kinetics27, as might stress caused by axotomy and the continual growth of primary cultures. Intravital analysis of axonal transport of individual cargoes (Figure 1) is therefore likely to provide more physiologically relevant insights into this dynamic process, albeit with its own pitfalls (Table 1)28–30.
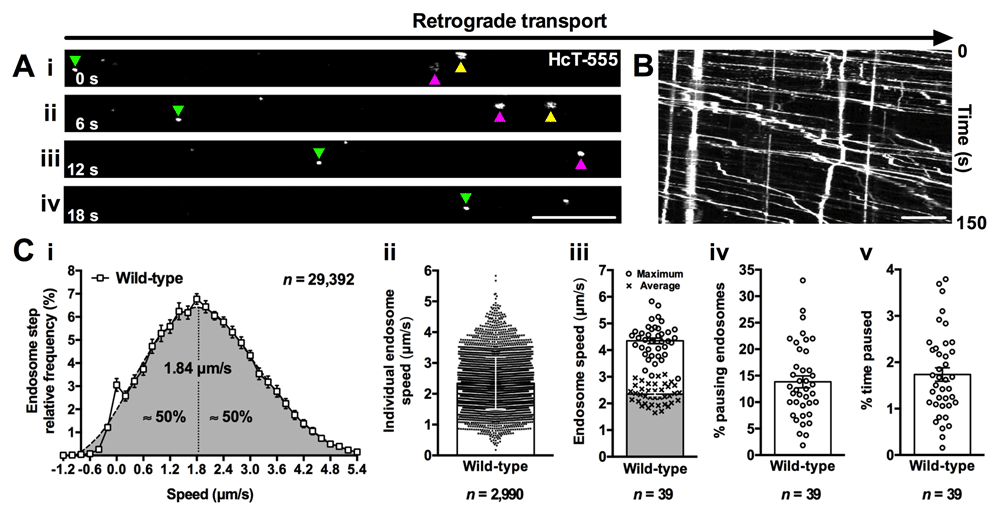
(A) The transport of individual fluorescent cargoes along axons can be assessed in vivo. This series of time-lapse confocal microscopy images (i-iv) depicts the retrograde movement (left to right) of the tetanus toxin binding domain (HcT-555) in sciatic nerve axons, as detailed in Gibbs et al.11. Distinct signalling endosomes (e.g. coloured triangles) loaded with HcT-555 can be tracked across multiple images and transport assessed. (B) Representative kymograph generated from fluorescently labelled signalling endosomes being transported in sciatic nerve axons. (C) Numerous features of axonal transport kinetics can be assessed and plotted (see Box 3 also); for example, speed distribution curves of individual endosome steps (i), average endosome speeds (ii), mean endosome speeds per animal (iii), the percentage of endosomes that remain stationary for at least two consecutive frames (iv), and the percentage of time spent pausing (v). The data reported here were generated from 39 wild-type (C57BL/6) animals aged from 1 to over 13 months, which is a period when transport dynamics are known to remain stable71. Scale bars (A–B) = 10 μm.
In this short review, we will highlight recent methodological advances and adaptations enabling the in vivo imaging of axonal transport across model organisms, specifically focusing on techniques that track the fast axonal transport of individual cargoes in real-time rather than transport en masse. We will outline strengths and weaknesses of the methods along with findings they have generated (Box 1), highlight major outstanding questions (Box 2), and discuss possible improvements and future directions for in vivo analysis.
Consistent with sciatic nerve explant data5, the percentage of motile mitochondria in the Drosophila marginal nerve declines with age, while the dynamic properties (run speed and length) remain unchanged45
Reduced levels of the dynein co-factor Lissencephaly-1 caused an increase in the percentage of motile mitochondria in the Drosophila marginal nerve45
Defective mitochondrial transport results in an increase of protein aggregation in Drosophila neurons; conversely, upregulating mitochondrial transport correlates with a delayed appearance of protein aggregates45
In zebrafish retinal ganglion cells (RGCs), disruption of Kif5A, a neuron-specific anterograde motor, resulted in increased frequency of retrograde mitochondrial transport but not synaptophysin-containing vesicles58
Zebrafish models of Charcot-Marie-Tooth disease type 2A59 and Parkinson’s disease60 showed mitochondrial motility defects in disease-relevant nerve cells
During larval development (2–5 days post-fertilisation), the percentage motility of mitochondria and the ratio of anterograde to retrograde movements progressively decreased in zebrafish central nervous system (CNS) dopaminergic neurons, whereas run length increased, but speeds remained stable60
Amyotrophic lateral sclerosis (ALS) mice, but not spinal and bulbar muscular atrophy mice70, display pre-symptomatic defects in the transport speeds of signalling endosomes in sciatic nerve axons67; mitochondrial transport is also perturbed in both SOD and TDP-43 models of ALS67,68
Retrograde axonal transport kinetics (speed, % pausing, and % time paused) of signalling endosomes in the sciatic nerve remain unchanged from 1 to over 13 months71, which varies from the age-related transport deficiencies reported in different experimental settings at similar time points12,89,90
Bi-directional defects in the transport of both mitochondria and peroxisomes are detected in spinal cord axons of acute and chronic multiple sclerosis mouse models before major symptom onset77
In mouse RGCs, the number of moving mitochondria, but not run length, was decreased prior to cell death in a glaucoma model, whereas the duration and distance of mitochondrial transport were both diminished with age (23–25 months)12
The vast majority of mitochondria in neonatal and adult cortical pyramidal neurons remain stationary over periods of up to 20 minutes13,14
What is the direct biological significance of altered cargo pausing and transport speeds?
Are the defects in axonal transport observed in myriad neurological disease a cause of neuronal dysfunction or the consequences of a dysfunctional neuron?
Does ageing impact all neuronal and cargo subtypes equally?
Why do mutations in mitochondrial91 and motor proteins1 often manifest in a nervous system-specific pathology?
What causes the axonal transport of distinct cargoes to be differentially affected by ageing and disease-associated mutations?
The sophisticated genetic toolboxes of Caenorhabditis elegans and Drosophila melanogaster allow the specific targeting of fluorescent proteins to vesicles and organelles such as mitochondria. When coupled with the ever-expanding repertoire of neurological disease-relevant worm and fly models31–34, these reporter lines provide a powerful system for analysing the axonal transport of assorted cargoes in both ageing and disease35–40. Transport studies in Drosophila have largely been performed in filleted larval preparations rather than adult flies, limiting the time period over which analyses can be performed and the developmental stage of the neurons under investigation. Third instar larvae are typically dissected for imaging of fluorescent cargoes predominantly in motor axons41,42. Alternatively, microfluidic devices that physically immobilise intact Drosophila larvae afford a non-invasive approach to image axonal transport43,44.
A novel technique to analyse transport dynamics in sensory axons of the Drosophila wing has been developed (Figure 2A), which permits the assessment of axonal trafficking throughout the lifespan of adult flies45,46. The marginal nerve found on the anterior edge of fly wings consists of chemosensory and mechanosensory neurons47,48, the cell bodies of which are connected by short dendrites to wing bristles, while their axons bundle together and project to the central nervous system (CNS)45. Given the translucency of the wing and the accessibility of the marginal nerve, rapid and non-invasive light microscopy can be performed on different wing regions of flies expressing fluorescently tagged proteins specifically in neurons. Motivated by previous work in which the same nerve was used to evaluate in vivo responses to neuronal injury49,50, the GAL4-UAS system was implemented to visualise mitochondria and dense core vesicle (DCV) transport dynamics in flies up to 30 days old (their natural lifespan being ≈50 days in the laboratory)45. Flies showed an early peak in the number of bi-directional moving mitochondria during early adulthood and subsequent decline with age linked to misfolded axonal protein accumulations; however, the dynamic properties of the moving mitochondria did not change with time. Intriguingly, reduced levels of the dynein co-factor Lissencephaly-1 (Lis1) caused a doubling in the number of motile mitochondria across time-points (without increasing their number) and reduced the age-associated build-up of protein in the axon45. Contrasting with mitochondria, the percentage of motile DCVs remained steady across ages in wild-type flies and was unaffected by reduced Lis1 levels, which is indicative of an organelle-specific perturbation rather than a global transport defect. Although the Drosophila marginal nerve cannot be used for whole-mount staining or large-scale biochemistry and has no direct counterpart in humans, its simple preparation permits quick and non-invasive analysis of anterograde and retrograde axonal transport in sensory nerves of adult flies. Without constraints on fly age, extended experimental time-points can be incorporated, facilitating the study of ageing and neurodegeneration in Drosophila.

(A–C) Recent technical advances have permitted the assessment of axonal cargo dynamics in a range of neuron types across different live model organisms. In the past few years, organelles have been tracked for the first time in sensory neurons of the adult Drosophila wing (orange, A)45,46 and larval zebrafish retinal ganglion cells58, central nervous system (CNS) dopaminergic neurons (red, B), and middle primary (MiP) motor neurons (blue, B)59,60. In the mouse, a more experimentally challenging animal model because of its non-translucency, in vivo transport was assessed in (i) motor and sensory sciatic nerves11,68,71,72, axons of the spinal cord and dorsal roots77, (ii) retinal ganglion cells12, and (iii) distal layer 1–3 cortical pyramidal neurons13,14 (C). The purple, dashed-line boxes indicate approximate imaging regions.
As a genetic model, the zebrafish (Danio rerio) possesses many of the advantages of the invertebrate species, such as short generation time and lower maintenance costs, with the added benefit of being a vertebrate with myelinated axon tracts51. Also, like worms and flies, zebrafish are highly genetically tractable, with an array of reporter lines expressing fluorescently tagged proteins in specified organelles in subsets of cells. Zebrafish larvae are translucent, which obviates the need for invasive imaging techniques and allows repeated, longitudinal in vivo measurements throughout development. Several studies have probed cargo movement in the axons of anaesthetised zebrafish, the first of which assessed mitochondrial dynamics in sensory nerves called Rohon-Beard (RB) neurons found in the tail tip52. These transgenic “MitoFish” were created using the GAL4-UAS system to specifically express fluorescent proteins in the mitochondria of single RB neurons. A number of other groups have created similar fluorescent fish to assess the dynamics of RB mitochondria53 and endosomes54,55, as well as lysosomes in mechanosensory axons56,57.
Until recently, the bulk of in vivo zebrafish transport analysis was conducted in sensory nerves; however, a number of groups have capitalised on the translucency and genetics of the fish and expanded the arsenal of neuronal types amenable to imaging. To assess the visual system, transgenic fish expressing fluorescent protein in either the synaptic vesicles or the mitochondria of retinal ganglion cells (RGCs) were created58. Disruption of the neuron-specific anterograde motor Kif5A increased the number of small motile synaptophysin-containing vesicles at early developmental stages without altering the ratio of anterograde to retrograde transport. In contrast, the percentage of mobile mitochondria was unaffected in Kif5–/– animals, but mitochondria were transported more frequently in the retrograde direction, which likely causes the observed depletion of axonal mitochondria. Similar to the above Drosophila study45, these experiments once again highlight that transport of distinct organelles can be differentially impacted depending on the type and stoichiometry of the motor proteins driving their movement. The results also emphasise the importance of measuring multiple transport parameters (Box 3), as different conclusions would have been reported if just the percentage of motile mitochondria was assessed. In addition to RGCs, new transgenic lines have been generated to assess mitochondrial dynamics in middle primary (MiP) motor neurons of the spinal cord59 and CNS dopaminergic neurons60 (Figure 2B). These fish were used to show that models of Charcot-Marie-Tooth disease type 2A (CMT2A)59 and Parkinson’s disease (PD)60 display perturbations in mitochondrial motility in disease-relevant neuronal subtypes; CMT2A motor nerves had a reduced percentage of motile mitochondria with unchanged speeds, while PD dopaminergic neurons displayed multiple transport defects dependent on the dose of toxin (MPP+) used to model the disease. The percentage of motile mitochondria and the ratio of anterograde to retrograde movements decreased during larval development (2–5 days post-fertilisation) in wild-type dopaminergic neurons, while speeds remained stable and run lengths increased60. Given the assortment of neurons now available for imaging, zebrafish provide an exciting platform for the in vivo analysis of axonal transport during development, with the caveat that once zebrafish reach adulthood, they become opaque, abrogating their utility for post-larval analyses.
A multitude of subtly different and sometimes co-dependent axonal transport parameters may be measured. Some features, such as the ratio of anterograde to retrograde movements, cannot be assessed for certain cargoes, e.g. signalling endosomes, which are transported only from the periphery to the cell soma. Cargo type should thus be considered and the aims of each individual experiment carefully determined before selecting from the following analysis options, which may also be subdivided into anterograde, retrograde, bi-directional, and combined categories:
Speed
Frequency of frame-to-frame speeds (Figure 1Ci)11,67,70,71
Individual cargo average velocities (Figure 1Cii)71
Average52,56,71,92 and maximum cargo speeds (Figure 1Ciii)71
Immobile cargoes can be either included or omitted, and analysed separately59, while movement-only speeds (i.e. uninterrupted runs or constant-velocity segments) can be determined52,92
Motility
Pausing
Anterograde, retrograde, and bi-directional
Microscope settings can also impact axonal transport results, so care must be taken when making cross-study comparisons. Furthermore, whether recordings will be manually or automatically tracked must also be taken into account. The following should therefore be contemplated:
Frame rate: there is always a trade-off between sampling frequency and specimen integrity93,94. For example, a low-frequency frame rate (less than 1 Hz) could miss brief pauses, resulting in the recording of slower “moving” speeds for individual cargoes and fewer pauses. Rapid frame rates may provide more accurate information but must be offset against how rapidly a sample bleaches, potential phototoxic changes to specimen physiology, and the signal-to-noise ratio. Frame rate also directly impacts the labour required for analysis if manual tracking of cargoes is carried out.
Imaging time: the longer an axon is imaged, the greater the chance that stationary organelles, particularly mitochondria, will become motile. The impact of phototoxicity should also be considered. Imaging over several orders of magnitude can circumvent this problem: for example, imaging at 2 Hz for 1 minute followed by 0.2 Hz for 10 minutes95.
Intravital imaging techniques have been developed in mice to study a range of biological processes in vivo, including the response to spinal cord injury61, retinal degeneration62, and cortical function and development63,64. Similar to the animal models mentioned above, experimentalists working with mice can use an extended library of transgenic fluorescent reporter strains facilitating live imaging28,65. Consequently, there has been a recent flurry of publications adapting these in vivo techniques for the analysis of axonal transport in live mice.
Among these, transgenic mice selectively expressing fluorescent proteins in neuronal mitochondria called “MitoMice” were generated by the Lichtman laboratory in 200766. Mitochondria are labelled throughout most of the “MitoMouse” nervous system, permitting in vivo analysis across a broad spectrum of neuronal subtypes provided they can be accessed in live animals. In this publication, in vivo axonal transport of individual organelles was imaged for the first time in a live mammal66. Mitochondrial kinetics were analysed in single motor and sensory axons of surgically exposed sciatic nerves using time-lapse confocal microscopy (Figure 2Ci).
Adaptations of this technique have since been developed and expounded upon by a number of different laboratories. The Schiavo group crossed the “MitoMouse” with the SOD1G93A mouse model of ALS and showed that mitochondrial transport speeds are pre-symptomatically impaired in sciatic nerve axons, which is one of the first observable deficiencies in this disease model67. Another laboratory confirmed this result and expanded it to the TDP-43A315T mouse model of ALS68. Using a fluorescently tagged atoxic binding fragment of tetanus neurotoxin (HCT), the dynamics of a second type of organelle, the signalling endosome, were assessed in the sciatic nerve of SOD1G93A mice (Figure 1)67. Fluorescent HCT was injected under anaesthesia into the gastrocnemius and tibialis anterior muscles of the lower leg, where it binds to nidogen receptor proteins of the basement membrane before internalisation at the neuromuscular junction69. Once in the nerve terminal, the toxin hijacks the retrogradely transported signalling endosomes, which can be tracked in sciatic nerve axons in vivo11. Signalling endosome movement was also shown to be impaired67, suggestive of a generalised transport defect in SOD mutant mice. This is most likely caused by global changes in general transport machinery, e.g. the microtubule network, as opposed to disruption of multiple cargo-specific pathways. Confirming that defective retrograde transport of signalling endosomes is not a general read out of an impaired or aged nervous system, in separate studies spinal and bulbar muscular atrophy mice70 and wild-type animals aged to over 13 months71 were both shown to have normal endosome transport dynamics. A minor drawback of these studies is that the identity of sciatic nerve motor and sensory axons cannot be readily differentiated. Nonetheless, there is the possibility of injecting HCT into the footpad to target nociceptive sensory neurons or injecting a fluorescently labelled p75NTR neurotrophin antibody into muscle, which is mainly taken up by sensory nerves11,67. Moreover, crossing of disease models with transgenic mice selectively expressing fluorescent proteins in motor axons (e.g. using the Hb9/Mnx1 promoter) could also help to overcome this issue. Alternatively, the mainly motor femoral nerve72 or primarily sensory sural72 or saphenous nerves20 could be imaged either in fluorescent reporter strains or by altering the HCT injection site to ensure fluorescent signalling endosome transport in the appropriate nerve. However, these alternative peripheral nerves are more technically challenging to image on an inverted microscope because of their anatomical distribution and size. Indeed, the sciatic nerve is a large, superficial collection of peripheral nerve axons, in which the dynamics of various cargoes can be imaged, with the useful possibility for longitudinal analysis in the same animal66,72.
In contrast, the mouse CNS is inherently more difficult than the relatively accessible peripheral nervous system to image directly; nevertheless, axonal transport has now been successfully tracked in a number of CNS neurons. Axons within the spinal cord can be surgically exposed by dorsal laminectomy and imaged across time and several spinal segments in vivo61,73–75, permitting longitudinal and location-specific comparisons. Mice expressing fluorescent proteins in only a small percentage of sensory neurons have been used to assess axonal degeneration caused by spinal cord lesion in individual large, myelinated sensory axons found in the dorsal aspect of the spinal cord61. Numerous synthetic vital dyes that label structures such as myelin and microglia can also be used to aid in vivo analysis of the spinal cord76. This technique was recently implemented to assess axonal transport in acute and chronic mouse models of multiple sclerosis (MS)77. Crossing these strains with “MitoMice” and specifically generated “Thy1-PeroxiYFP” mice, both mitochondria and peroxisomes, respectively, were imaged in spinal cord and dorsal root axons. Pervasive defects in anterograde and retrograde transport of both cargo types were observed in MS mouse spinal cord axons before the onset of additional deficiencies, suggesting that axonal transport defects represent an early and important pathological sign in this disease77. These defects were not seen in dorsal root axons (sensory nerves), suggesting that subcellular location (i.e. proximity to the soma) has a bearing on axonal transport defects. However, the sensory identity of the axons imaged in the spinal cord was only presumed (owing to their dorsal location) and not experimentally confirmed, so the potential site-specific transport issue could instead be a product of neuron subtype (e.g. motor versus sensory).
Multi-photon microscopy has also been used to assess mitochondrial transport dynamics in the axons of RGCs of anaesthetised “MitoMice” (Figure 2Cii)12. RGC axons extend into the nerve fibre layer of the eye, parallel to the ocular surface of the sclera, permitting the visualisation of organelles after subtle opening of the surrounding skin and conjunctiva. The number of moving mitochondria, but not their run length, was diminished before RGC death in a model of glaucoma, whereas in aged mice (23–25 months), the duration and distance of mitochondrial movement were both diminished12. Interestingly, counter to results from retinal explants in the same study, this method showed that mitochondrial transport is highly dynamic in the mouse CNS in vivo12. Unfortunately, as fluorescence microscopy could impact the activity of light-sensitive retinal neurons, this observation may be confounded by artefactual alterations in mitochondrial dynamics (i.e. caused by increased activity20). Furthermore, this technique is restricted to albino strains because uveal pigmentation prevents imaging, and is more technically challenging with gaseous anaesthetics because of face access requirement. Nonetheless, in vivo comparisons of organelle transport in proximal and distal regions of RGC axons allow for repeated, longitudinal analyses.
Finally, a technique developed to image distal layer 1–3 pyramidal neurons of the cortex has been independently adapted by two groups to assess axonal transport through a surgically fitted cranial window in both anaesthetised and awake mice (Figure 2Ciii)13,14. Rather than using transgenic fluorescent strains, plasmids were unilaterally electroporated into embryos in utero to target the expression of fluorescent proteins to both the mitochondria and the cytoplasm13 or membranes14 of cortical progenitor cells (the latter to aid the identification of successfully transfected neurons). The process of electroporation restricts the fluorescence to a small proportion of axons, facilitating the assessment of individual collaterals. Similar to previous in vivo reports from zebrafish RB sensory52 and CNS dopaminergic60 neurons and mouse sciatic nerve axons66, the percentage of immobile mitochondria in cortical axons was very high over short time periods: >99% in 2 minutes in both pups (P10–13) and adults (P70–120)14 and ≈90% at P10–12 over 10–20 minutes13. The discrepancy between these two studies could reflect the different but overlapping cortical layers that were imaged; however, it is more likely caused by the 5- to 10-fold difference in imaging periods. Indeed, over 95% of mitochondria were reported as stationary in mature cortical neurons in culture when imaged for 30 minutes, which drops to ≈75% using a 12-hour imaging window13. This highlights a key problem in the study of axonal transport: to track individual cargo movements, rapid frame rates are required, but biologically relevant transport events may occur separated by much longer periods (minutes to hours), which is particularly challenging for in vivo imaging (Box 3). Thus, although surgically intensive and technically demanding, this method of imaging cortical collaterals allows repeated, longitudinal analyses of CNS neurons of the brain in live, non-anaesthetised mice.
The active transport of organelles and molecules along axons is critical to neuronal health, function, and survival. Deficiencies in this process appear to be intricately linked to ageing and neurodegeneration, but whether they play a causal role or are simply a consequence of a pathologically affected tissue remains to be fully elucidated in each setting (Box 2). There are numerous possibly conflicting data reported on the dynamics of axonal cargoes. These differences could be due to several reasons, including experimental setting and parameters, neuronal subtype, cargo type, time-point, axonal location (i.e. distal versus proximal78), and neuronal morphology (e.g. proximity to axonal arbor branches52). With recent developments discussed here, we are beginning to acquire a diverse and very powerful arsenal of in vivo experimental systems across model organisms that will greatly enrich our understanding of transport. It is now vital to implement these intravital methods to tackle the questions of when (age), where (neuron category and subcellular location), what (cargo type), and how axonal transport deficiencies manifest in neuronal dysfunction.
Progress in imaging axonal transport in vivo has challenges in common with all in vivo imaging experiments (e.g. limited transparency of tissue, restricted imaging depth, and phototoxicity) as well as formidable problems unique to the phenomena being studied: namely, relevant scales of measurement that span several orders of magnitude, both in time and distance. The difficulty in labelling cargoes specifically and with a labelling density allowing a sufficient signal-to-noise ratio for transport analysis is highlighted by the small number of axonal cargoes currently being studied, which are almost exclusively membranous organelles. Using bright, photoactivatable fluorophores is a particularly helpful strategy for studying transport, as it allows the tracking of subpopulations of cargo otherwise too dense to study individually or sparse cargoes over long time periods13. However, there is an urgent need to adopt new labelling strategies and fluorescent reporters to understand the behaviour of non-membrane-bound organelles, particularly in the field of RNA transport. Much of what has been learnt about organelle transport is garnered from experiments on mitochondria30; however, their movement within axons, which is characteristically interspersed by long pauses and thus perhaps more characteristic of slow axonal transport, is not reflective of all cargo types. This is perhaps because they have distinct axonal roles, rely on specific subsets of adaptor and motor proteins2, and are unique, highly dynamic, network-forming organelles. To have a thorough and informed understanding of axonal transport in health and disease, it is therefore of paramount importance that multiple cargo types are analysed.
Most of the methods discussed here are adaptations of intravital techniques initially developed to study other biological processes. It is therefore likely that imaging of additional neuronal subtypes or subcellular locations79–86 could be incorporated into these analyses in order to provide a more global assessment of in vivo axonal transport in health and disease. Moreover, as imaging techniques become more sophisticated, allowing high-speed, multi-channel acquisition at greater tissue depths87,88, we will be able to simultaneously monitor the dynamics of different organelles in their native environment and reliably assess the transport of organelles, such as RNA granules, for which similar robust protocols are currently lacking.
This work was supported by Wellcome Trust Sir Henry Wellcome Postdoctoral Fellowships (103191/A/13/Z to J.N.S. and 096141/Z/11/Z to A.E.T), a NC3Rs David Sainsbury Fellowship (NC/N001753/1 to A.V.), a Wellcome Trust Senior Investigator Award (107116/Z/15/Z to G.S.), and University College London (G.S.).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors would like to thank members of the Schiavo and Linda Greensmith (Institute of Neurology, UCL) laboratories for productive discussions.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 01 Mar 17 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)