Keywords
Vasomotor symptoms, hot flashes, menopause, galvanic skin response, GSR, electrodermal activity, EDA, physiological monitoring, health tracking
Vasomotor symptoms, hot flashes, menopause, galvanic skin response, GSR, electrodermal activity, EDA, physiological monitoring, health tracking
Continuous tracking of electrodermal activity (EDA), also known as galvanic skin response (GSR), values with commercial fitness devices for individuals with vasomotor symptoms (hot flashes) provides a path forward for future studies with fine resolution monitoring. This can improve upon the current reliance on the use of personal diaries (Regestein et al., 2015). There are multiple conditions associated with vasomotor symptoms including menopause/early menopausal transition (Hale et al., 2014), medications (Quinestrol, tramadol, etc.), chemotherapy and Tamoxifen, hyperthyroidism, infections (Inflammatory Bowel Disease – IBD, etc.), and more. Multiple studies have characterized hot flashes in premenopausal and menopausal women using self reported methods, laboratory polysomnographic recording, and some specially designed devices (Freedman, 2014). However, it is difficult for an individual to track and accurately report nighttime vasomotor symptoms without the aid of a physiological monitoring device (Freedman, 2014). Emerging commercial and custom devices with EDA meters will greatly facilitate the nighttime monitoring of hot flashes for individuals for more informative longitudinal studies of conditions with associated vasomotor symptoms. This report illustrates the potential fine-resolution monitoring of nighttime vasomotor symptoms using commercially available activity-tracking devices with an EDA sensor.
MIT Lincoln Laboratory conducted a longitudinal study on volunteers wearing physiological monitors (June 15 2014 to October 2 2016). The study protocol and written consent form were reviewed and approved by the MIT Committee on the Use of Humans as Experimental Subjects (COUHES). The commercial devices in the study include the Basis B1 watch and Basis Peak watch monitors for tracking heart rate, sleep with predicted sleep phases (light, deep, and REM – rapid eye movement), activity, skin temperature, and perspiration (EDA/GSR) without the use of electrodes or gel. The Basis B1 and Peak watches are no longer commercially available, but similar devices with EDA/GSR sensors are available.
Vasomotor symptoms started disrupting the sleep of a female volunteer on November 23, 2015, calling attention to their occurrence. After November 23, 2015, the volunteer started personally logging the occurrence of vasomotor symptoms, noting that the recorded EDA signals reflected the logged hot flash intensities and durations.
Figure 1 shows data from eight days around this time period. Freedman (Freedman, 1989) identified a good agreement between an increase of 2 μS/cm in 30 s time period with volunteer self-reports for vasomotor symptoms. For November 15th, the EDA median value was 6.8e-4 μS/cm and average value of 4.9e-2 4 μS/cm. For November 16th, the EDA median value was 6.8e-4 μS/cm and average value of 1.9e-2 μS/cm. Across all nights tracked, the EDA median value was 3.7e-4 μS/cm illustrating baseline EDA values for this volunteer. The longitudinal data collected indicates that the volunteer’s vasomotor symptoms may have been occurring as early as June 2014, but went unnoted until higher intensity vasomotor symptoms caused sleep disruptions. A review of all sleep time data indicates an increase in sleep interruption minutes as reported by Basis (52/1957=2.9% for EDA range 10-15 μS/cm and 15/311=4.8% for EDA range 15-25 μS/cm compared to 3823/258119=1.5% for EDA < 1 μS/cm). This is consistent with volunteer observations. Nights like November 23 and 24, 2015 cause sleep disruptions. Figure 2 illustrates over two years of nighttime EDA values while this volunteer was sleeping. Starting in December 2015, the volunteer started self-tracking EDA values and vasomotor symptoms. Daytime peaks were associated with both exercise and vasomotor symptoms. The volunteer reports that daytime vasomotor symptoms and sleep-disrupting vasomotor symptoms were consistent with recorded EDA peaks but they did not record these observations. Nighttime EDA peaks well above baseline values were observed in clusters from June 2014 until November 2016. Note that nights with low EDA values still occur frequently for this volunteer, indicating nights free of vasomotor symptoms. The volunteer did not take hormone or nonhormonal formulations for the treatment of vasomotor symptoms. Note that the volunteer’s Basis B1 watch was replaced with a Basis Peak watch in August 2015.
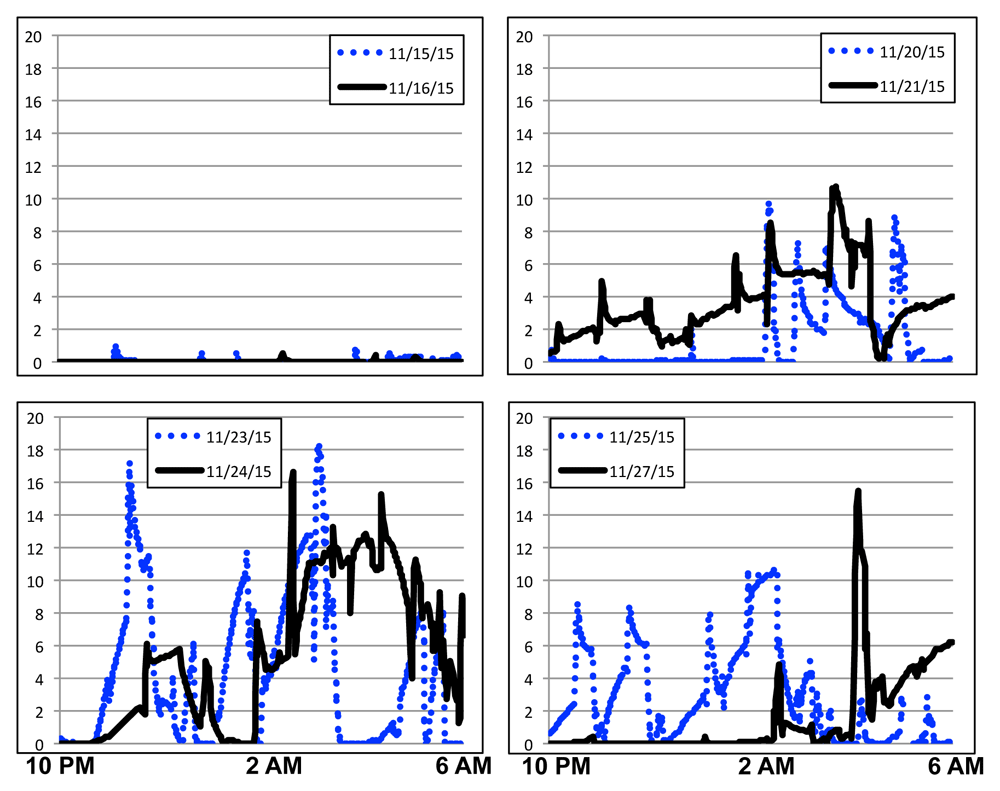
Vertical axis shows EDA values in μS/cm.
Longitudinal studies of large numbers of volunteers will provide new foundations for tracking vasomotor symptoms associated with menopause and other conditions. Continuous tracking of EDA values with readily available commercial tracking devices will provide a path forward for future fine-resolution longitudinal studies of conditions associated with vasomotor symptoms. Insights into understanding and treating vasomotor symptoms will be greatly advanced by these longitudinal studies, as the different causes of vasomotor symptoms may vary in intensities and durations. EDA is also reported as a sensitive index of sympathetic nervous system activity (Poh et al., 2010). In addition, commercial devices with EDA meters will be valuable personal monitoring tools to premenopausal and menopausal women and individuals experiencing vasomotor symptoms.
The nighttime EDA values tracked are available in Ricke, Darrell, 2017, "GSR nights", doi: 10.7910/DVN/ONAUUZ, Harvard Dataverse.
Written informed consent was obtained from the volunteer for the publication of her details.
This material is based upon work supported by the Assistant Secretary of Defense for Research and Engineering under Air Force Contract No. FA8721-05-C-0002 and/or FA8702-15-D-0001. Any opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Assistant Secretary of Defense for Research and Engineering. Assistant Secretary of Defense for Research and Engineering has no involvement in this report.
The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
I thank Emily Simons for graphics layout support and Paula Collins for careful review of this manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Newton KM, Carpenter JS, Guthrie KA, Anderson GL, et al.: Methods for the design of vasomotor symptom trials: the menopausal strategies: finding lasting answers to symptoms and health network.Menopause. 2014; 21 (1): 45-58 PubMed Abstract | Publisher Full TextCompeting Interests: I am not involved with any research or companies specifically exploring the use of objective hot flash monitoring. However I work with 2 companies who are developing treatments for hot flashes: 1. Menogenix: Scientific Advisory Board and stock options 2. Ogeda/Astellix: Scientific Advisory Board
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 20 Dec 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)