Keywords
cyanobacteria, nitrogen fixation, freshwater, marine
cyanobacteria, nitrogen fixation, freshwater, marine
Nitrogen fixation, the biochemical conversion of “inert” atmospheric N (N2) to biologically available ammonia (NH3), is a microbially mediated process of global significance because it provides “new” N to aquatic ecosystems in which biological production is often controlled by N availability1,2. N2 fixation is an anaerobic process carried out by specific prokaryotes, including heterotrophic and chemolithotrophic bacteria and some cyanobacteria (blue-green algae)3. The process likely evolved during the oxygen (O2)-devoid Precambrian period some 2+ billion years ago4,5. Of the N2-fixing microbial taxa, the cyanobacteria are of particular biogeochemical and ecological interest because they were also the first O2-evolving photosynthetic organisms on Earth6; their proliferation during this period is thought to be an evolutionary “milestone” because it led to the generation of an O2-rich atmosphere, a prerequisite for the evolution of O2-requiring fungi, bacteria, animals, and higher plant species on our planet6.
Ironically, the development of an O2-rich atmosphere, hydrosphere, and pedosphere constituted a formidable biochemical challenge for the cyanobacteria because, while they were capable of fixing N2, the process had to be confined to an O2-free micro-environment7. This requirement posed a serious dilemma, especially for aquatic cyanobacteria, because they require illuminated conditions in surface waters, but the high ambient O2 levels produced by photosynthesis in these waters also represents an environmental barrier to O2-sensitive N2 fixation. Over their long evolutionary history, cyanobacteria have developed biochemical and structural adaptations as well as biotic associations in order to optimize N2 fixation while relying on oxygenic photosynthesis to provide energy and organic carbon (C) compounds to support metabolism and growth. The adaptions include (1) confining N2 fixation to night-time when photosynthesis is “turned off”, (2) forming colonies and aggregates to reduce illumination and form low-O2 “microzones”, (3) participating as endosymbionts in biological associations, and (4), forming heterocysts (non-photosynthetic, O2-free cells) in some filamentous taxa, which allows N2 fixation to proceed while receiving photo-reductant and organic C through photosynthesis from adjacent cells8.
These are all remarkably clever adaptations to a modern-day oxic biosphere, which help circumvent the “O2 problem”6. From an ecosystem perspective, they have allowed N2-fixing species to provide biologically available N from the vast reservoir of atmospheric N2. However, on the ecosystem scale, recent N budget analyses indicate that N2 fixation inputs fall far short of meeting ecosystem requirements when biologically available N inputs (from terrestrial and atmospheric sources) and losses (via denitrification, sedimentation and burial, and advection) are considered9–11. As a result, freshwater, estuarine, and marine systems are often chronically N deficient11–17. Pervasive N limitation has many implications for ecosystem function, especially when excessive external nutrient inputs lead to accelerating primary production (eutrophication), harmful algal blooms, and excessive O2 consumption (hypoxia). If chronic N-limited conditions prevail in water bodies and N2 fixation cannot meet ecosystem N requirements, then external N inputs often supply N to support eutrophication and its unwanted symptoms. From a management perspective, this means that the growing global glut of N inputs from agricultural, urban, and industrial sources14,18–20 needs to be controlled, in addition to the broadly accepted phosphorus (P) input constraints, in order to protect our waterways and water supplies.
Why does N2 fixation fall short of meeting ecosystem demands? Apparently, this process does not operate at sufficient rates in a modern-day, oxic world to compensate for losses via burial, export, and denitrification, even though it is protected and optimized by the various biological adaptations mentioned above. It is counteracted at larger scales by biogeochemical processes, such as denitrification, that run in the opposite direction (NO3 → N2). The N2-fixing process is an energy-demanding one, requiring 16 ATP molecules to fix one molecule of N23. In cyanobacteria, this energy demand has to be met by photosynthesis, while in non-photosynthetic bacteria, organic matter and redox reactions serve as energy sources3. In highly productive (eutrophic), turbid waters where cyanobacteria and bacteria thrive, the availability of photosynthetically active radiation (PAR: 400–700 nm) is often restricted, causing a radiant energy deficit and suboptimal N2 fixation rates. Secondly, cyanobacteria taxa that dominate in eutrophic waters often accumulate as thick surface “blooms”, in part to circumvent light limitation in subsurface waters11. High rates of photosynthesis in such blooms lead to O2 supersaturation, often in excess of 200% saturation21. These ambient O2 levels inhibit N2 fixation in situ, even in heterocystous taxa22,23. Thirdly, N2 fixation requires high levels of P (to support the energetics, e.g. ATP formation and nucleic acid production) and metals, most prominently iron (Fe), which is a co-factor in the enzyme complex nitrogenase3. In highly oxygenated surface waters, Fe occurs as the insoluble and biologically unavailable Fe3+ ion that may lead to Fe-limited conditions24. Lastly, wind-induced turbulence and vertical mixing can reduce N2 fixation potential by disrupting colonies and aggregates and enhancing inward diffusion of O2 (Figure 1)25 and deepening the mixed layer, reducing light availability.
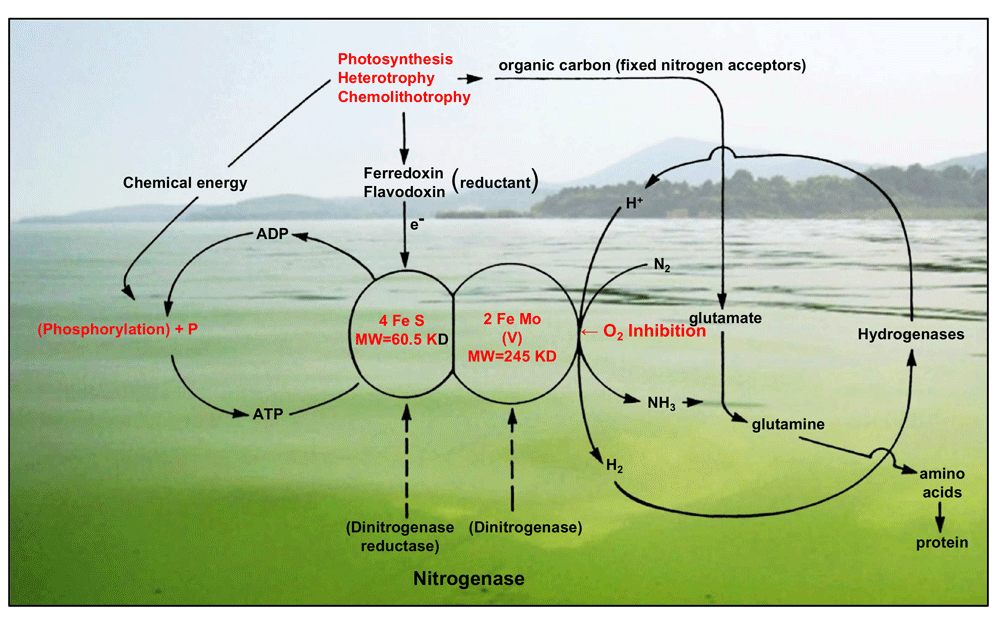
Potential environmental controls, including phosphorus (P) and iron (Fe) availability, energy sources, and dissolved oxygen inhibition, are shown in red. The background photo is of an O2-supersaturated (during daytime) cyanobacterial surface bloom in Lake Taihu, China. Photograph by H. Paerl.
Thus, while N2 fixation converts inert N2 into biologically available NH3 to support aquatic fertility in a remarkable fashion, it faces multiple constraints and limitations in aquatic environments, especially in surface waters, which are often N limited. Geochemists, some limnologists, and a few oceanographers have assumed that as long as P and Fe are readily available, N2 fixation should make up for an N deficit, given the unlimited supply of N2 available26,27. However, this assumed linear stoichiometric relationship is not straightforward. Major environmental factors constrain this process, preventing it from functioning at optimal rates and supplying complete ecosystem N requirements8,11. As a result, much of the world’s marine and freshwater environments remain chronically N deficient. In practical (management) terms, this limitation means that external inputs of N play a key role in providing adequate and excessive fertility (eutrophication) of many freshwater and most marine ecosystems11,15,16. Tremendous increases in anthropogenically generated bioavailable N in the form of synthetic (Haber process) fertilizers, agricultural, industrial, and urban wastes, and N2 emissions (as both oxides and reduced forms of N) far overshadow biological fixation of N2 in providing available N to receiving waters. Effective future management and protection of our fresh and marine waters will depend on the control of external inputs of both N and P11,27 instead of depending on the more traditional approach of controlling P inputs without N restrictions28.
This work was partially supported by the National Science Foundation (DEB 9815495; CBET 0826819, 1230543; and Dimensions of Biodiversity 1240851).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
I appreciate the helpful comments by my colleagues W. Gardner, M. McCarthy, and J.T. Scott and appreciate the technical assistance from A.R. Joyner.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 09 Mar 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)