Keywords
HIV, tuberculosis, early intervention, sub-Saharan Africa
HIV, tuberculosis, early intervention, sub-Saharan Africa
Co-infected patients with HIV and tuberculosis (TB) has been a serious concern to healthcare sectors in many countries, commonly countries with resource constrained settings (Blanc et al., 2011; Manosuthi et al., 2012; Sinha et al., 2012). The incidence population of TB globally in 2012 was reported by the World Health Organisation (WHO) to be 8.6 million, and 1.1 million of this population were HIV-infected individuals (Mfinanga et al., 2014; WHO, 2013). The most challenging issue physicians are facing is the appropriate timing of introducing antiretroviral therapy (ART) along with ongoing TB therapy in HIV and TB co-infected patients (Mfinanga et al., 2014; Sinha et al., 2012). Delaying the introduction of ART for co-infected patients, and prescribing antibiotics only to these patients, has been proven to increase the risk of reactivation and reinfection of TB among patients, as a result of the HIV infection (Daley et al., 1992; De Cock et al., 1992; Sinha et al., 2012; Wilkinson & Moore, 1996). Hence it increases the death rate among co-infected patients, compared to individuals infected only with TB (Sinha et al., 2012; Wilkinson & Moore, 1996). The combination of the two therapies (ART and antibiotics) has been reported to have a significant outcome in reducing the mortality among co-infected patients, leading to a 90% reduction of TB reinfection (Manosuthi et al., 2006; Sanguanwongse et al., 2008). However, combining the two therapies is complicated, and can lead to drug-drug interactions, severe toxicities, poor medication adherence, increase pill burden, and risk of developing immune reconstitution inflammatory syndrome (IRIS) associated with TB (Mfinanga et al., 2014). To date, the cost effectiveness of early versus delayed ART initiation in co-infected patients has not been reported. The aim of this study is to examine the cost effectiveness of early versus delayed ART initiation in TB patients infected with HIV (co-infected patients).
Analytical decision model, comparing the early versus delayed ART initiation in co-infected patients, using cost utility analysis and cost effectiveness analysis.
A decision tree model was developed to compare the impact of early introduction of ART to delayed ART in the management of tuberculosis patients infected with HIV, and their mortality rate using Microsoft Excel 2013 (Figure 1). Though both conditions are chronic and as such a Markov model would also have been an appropriate tool to use (Soto, 2002). However, since the interested outcome can be assessed within a short time period (12 weeks), the decision tree model was used (Halpern et al., 1998).
The cost-effectiveness model was performed from the health-care’s payer perspective, where only the direct programs and medical costs were included. Indirect costs incurred by patients were not considered.
The study time horizon, used for the model is 12 weeks, and this was chosen based on the treatment period of latent TB, which can be 12 weeks (Manosuthi et al., 2012; Sinha et al., 2012).
The patients with TB, commencing on anti-TB treatment for 12 weeks, infected with HIV, are the population of interest. The setting was in sub-Saharan Africa. The data used in the model for both costs and consequences was extracted from previous published literature (summarised in Table 1; Abimbola et al., 2012; Cleary et al., 2006; Esfahani et al., 2011; Holland et al., 2009; Uthman et al., 2015). We conducted focused searches for the studies in Medline (from inception to December 2016) using the following keywords: tuberculosis, HIV, cost, and quality of life. Probabilities derived from published literatures were used for each arm on the tree to determine the number of patients that will either have adverse events or no adverse events, and those that will either survive or die.
ART, antiretroviral therapy; QALY, quality-adjusted life year.
| Parameters | Early ART | Delayed ART | Distribution | Reference |
|---|---|---|---|---|
| Probabilities | ||||
| Adverse event | 0.18 (18%) | 0.1 (10%) | Beta | (Uthman et al., 2015) |
| Death from adverse event | 0.24 (24%) | 0.27 (27%) | Beta | (Uthman et al., 2015) |
| Death from no adverse event | 0.08 (8%) | 0.09 (9%) | Beta | (Uthman et al., 2015) |
| Cost, $ | ||||
| ART treatment cost (patients that died within 3 months) | 320 | - | (Abimbola et al., 2012) | |
| ART treatment cost (patients that survive) | 350 | - | (Abimbola et al., 2012) | |
| Tuberculosis treatment with adverse event | 908 | 908 | (Esfahani et al., 2011) | |
| Tuberculosis treatment without adverse event | 868 | 868 | (Esfahani et al., 2011) | |
| Additional cost for dying patients | 832 | 832 | (Cleary et al., 2006) | |
| Utilities | ||||
| QALY of adverse event | 0.5 | 0.5 | (Holland et al., 2009) | |
| QALY of no adverse event | 0.81 | 0.71 | (Holland et al., 2009) |
Probabilities: Probabilities derived from published literatures were used for each arm on the tree to determine the number of patients that will either have adverse events or with no adverse events, and those that will either survive or die.
Utilities: The utility values, Quality adjusted life year (QALY) for each arm of the tree were also derived from published literatures.
Costs: Direct costs were the only cost considered in the model, as the perspective was based on health care only. The costs include costs of a complete treatment of TB with adverse or no adverse events (Esfahani et al., 2011) and costs of ART and costs of additional treatments for dying patients (Table 1). All costs were converted to US dollars ($), and inflated to 2014 price, using a USA inflation calculator (http://www.usinflationcalculator.com/).
Reduced death/mortality benefit was the primary outcome measured, and the incremental cost effectiveness ratio (ICER) was used, measured in QALY. The result will be presented on cost effectiveness plane (CE-plane), cost-effectiveness acceptability curves (CEACs), along with probabilistic sensitivity analysis (PSA) to represent the uncertainty in model output.
The following assumptions were made:
TB treatment - all patients were assumed to be undergoing anti-TB treatment that takes 3 months for completion (Holland et al., 2009).
Total ART treatment - it was assumed that the total ART treatment for 12 weeks is half the cost of healthcare utilization of patients that died within the first 6 months of ART. Also the same assumption was used for the patients that survive the first 6 months of ART. The cost excluded the expected expenditure per ART of included patients.
Mortality rate - it was assumed that the relative risk is three times more in patients with an adverse event group, than in a non-adverse group (Hoyo-Ulloa et al., 2011).
Additional costs of dying patients - it was assumed that the additional cost of treating dying patients was a result of the adverse event developed by the patients, which can result in death.
To handle uncertainty surrounding the model parameters, and the robustness of the model outcome, probabilistic sensitivity analysis was carried out to justify the decision on whether starting ART early or delaying treatment in TB patients infected with HIV is cost effective. About 10,000 random variables were generated, using the Microsoft Excel 2013 random generator. Cost effectiveness acceptability curves (CEAC) can also be used to summarize the uncertainty around cost effectiveness analysis (Fenwick et al., 2006). CEAC shows the probability of how cost effective an intervention is, compared with the alternative intervention, based on the range of threshold values accepted by decision makers per QALY (Fenwick et al., 2006).
Uncertainties around the cost effectiveness estimates can also be assessed using EVPI (Eckermann et al., 2010). Errors in cost effectiveness estimates can lead to wrong decisions, in which health benefit and resources can be forgone to an alternative choice (Briggs et al., 2006). The value cost of the forgone health benefit and resources as a result of uncertainty in the estimate, can be expressed as the EVPI (Briggs et al., 2006). EVPI can be expressed as the different association between the expected net benefit with no uncertainty and the expected net benefit with uncertainties. The value of EVPI rises as the threshold increases, as a result of the increment in decision uncertainty (Briggs et al., 2006). EVPI reaches the maximum when the value of threshold and expected ICER are equal, and this is the highest level of decision uncertainty (Briggs et al., 2006). The population EVPI was estimated by multiplying per patient EVPI by the effective population, i.e. the estimated number of people with TB and HIV co-infection. According to the WHO report in 2012, there were 1.1 million cases of co-infected patients, and 320,000 deaths were recorded among this population globally (WHO, 2013: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf).
Table 1 summarizes the model input parameters. The results of the analysis are shown in Table 2. From the tabulated results, the expected cost of providing early ART to TB patients infected with HIV was $1372, with a QALY gain of 0.68, while the cost of delayed ART was $955, with a QALY gain of 0.62. The results demonstrate that early ART provides a higher QALY value than delayed ART, but with a higher cost. The ICER shows $6775 per QALYs, which suggests that it is not cost-effective, since it is greater than 3 × GDP per capita ($5086) for the sub-Saharan Africa willingness to pay threshold (Evans et al., 2005; Murray et al., 2000).
ART, antiretroviral therapy; QALY, quality-adjusted life year.
| Strategy | Cost ($) | Incremental cost ($) | QALY | Incremental QALY | ICER ($/QALY) |
|---|---|---|---|---|---|
| Early ART | 1372 | 0.68 | |||
| Delayed ART | 955 | 417 | 0.62 | 0.06 | 6775/QALY |
The output for the probabilistic sensitivity analysis for 10,000 simulations is shown in Figure 2. All of the model outputs were in the northeast quadrant of the cost-effectiveness plane, suggesting that early ART is more costly and more effective than delayed ART; it is never cost saving and never has a negative impact on patient outcomes. At a threshold of $9,000, directly administered ART was found to be 50% more likely to be cost-effective, and if the willingness to pay for a QALY was $18,000 then directly administered ART is likely to be at least 95% cost-effective. The probability that early ART was cost-effective at the WHO-CHOICE threshold (Evans et al., 2005; Murray et al., 2000) of $5086 was just 1% (Figure 3). However, if the policy makers are willing to pay for a QALY ($10,000), then early ART is likely to be at least 95% cost-effective.
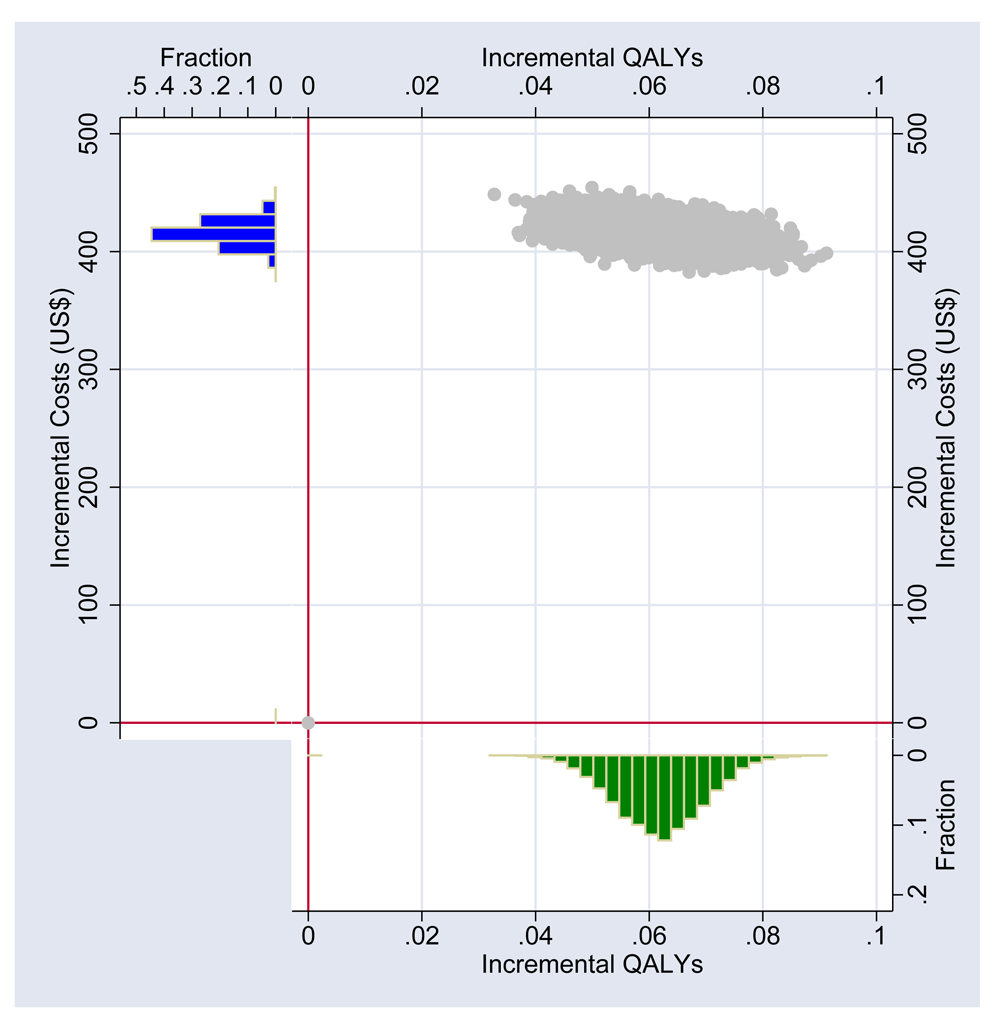
QALY- Quality Adjusted Life Years.
The population EVPI is illustrated in Figure 4. At a cost-effectiveness threshold of $5086, the population EVPI becomes substantial (≈$5 million), and is likely to exceed the cost of additional investigation. This suggests that further research will be potentially cost-effective. Co-infected early versus delayed antiretroviral therapy is unlikely to be cost-effective.
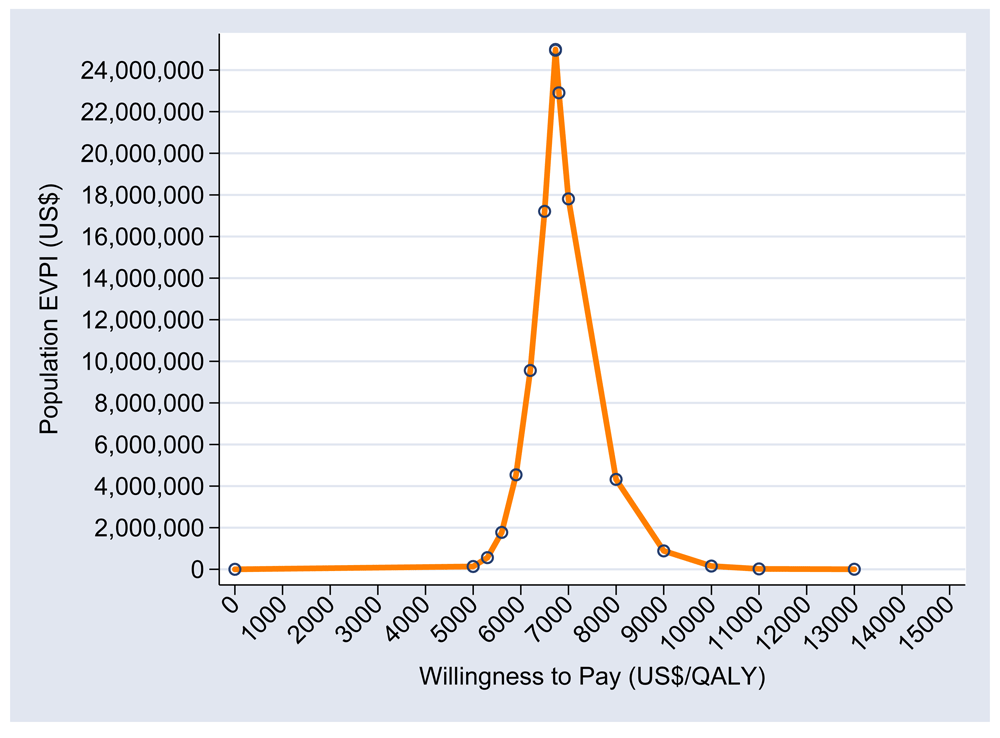
EVPI – Expected value of perfect information; QALY- Quality Adjusted Life Years.
The decision analysis model was used to assess the cost effectiveness of early ART in TB patients infected with HIV. According to the ICER estimates, early ART is not cost effective from a sub-Saharan Africa health-care payer perspective, i.e. the ICER is >3 × GDP per capita ($5086) (Evans et al., 2005; Murray et al., 2000).
To the best of our knowledge, this is the first cost-effectiveness model on optimal timing of ART in people with HIV and TB co-infection from sub-Saharan Africa’s perspective. Our cost-effectiveness model incorporated probabilistic sensitivity analysis to simultaneously and comprehensively estimate uncertainty around model input parameters. This approach follows WHO health economists' recommendations for economic evaluation and priority setting (Baltussen et al., 2002). In addition, the decision analytical approach we used have several advantages compared with economic evaluations alongside clinical trials (Ehlers et al., 2009). Evidence from multiple sources were combined, reflective of real-world evidence rather than evidence from just one trial conducted in a restricted setting. This can be combined and systematic sensitivity analyses performed (Ehlers et al., 2009).
The specific appropriate time to initiate ART within an early period could not be stated in the model, but it may be assumed that it should be within 8 weeks, as this is the recommended time by the WHO (Mfinanga et al., 2014). Only direct costs were considered in the model, based on the health care perspective. The costs, probabilities and utilities used in the model were estimated from published literature, and probabilistic sensitivity analysis was conducted to assess the uncertainties around parameter's value. The costs used seem to be general costs, which might not be the appropriate cost setting in sub-Saharan Africa.
In conclusion, from the perspective of the health-care payer in sub-Saharan Africa, early initiation of ART in HIV and TB co-infection cannot be regarded as cost-effective based on current information. The value of information analysis shows that further research will be worthwhile and potentially cost-effective in resolving the uncertainty about whether or not to start ART early in HIV and TB co-infection.
Dataset 1: Raw data for Figure 2, Figure 3 and Figure 4 (in zipped file). doi, 10.5256/f1000research.10620.d151708 (Uthman & Uthman, 2017).
RTU and OAU were responsible for conception and design of the research. Acquisition of data was carried out RTU and OAU. Economic modelling and statistical analysis were carried out by RTU and OAU. RTU and OAU were responsible for review, analysis and interpretation of the outcomes. RTU and OAU were responsible for development of the manuscript. RTU and OAU were responsible for critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: TB-HIV Treatment
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 13 Mar 17 |
read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)