Keywords
ventilator-induced lung injury, diaphragmatic dysfunction, mechanical ventilation,
ventilator-induced lung injury, diaphragmatic dysfunction, mechanical ventilation,
Respiratory failure is the leading cause of admission to pediatric intensive care units (PICUs)1–3. Mechanical ventilation (MV) is a lifesaving therapy, allowing the support of patients with respiratory failure with the objectives of improving gas exchange and decreasing work of breathing. MV consists of a pressurized volume of gas delivered by either an invasive (tracheal tube or tracheostomy) or a non-invasive interface. MV is particularly challenging in children because of the heterogeneity of this population in terms of age, weight, and pathophysiology.
In this brief review, we aim to discuss the current clinical challenges in pediatric ventilatory assistance outside of the neonatal patient population. We will focus this discussion on recent advances regarding 1) optimization and individualization of patient–ventilator interactions during MV, 2) application of high-frequency oscillatory ventilation (HFOV), and 3) the role of noninvasive ventilation (NIV) (Table 1 and Figure 1).

HFNC, high-flow nasal cannula; HFOV, high-frequency oscillatory ventilation; MV, mechanical ventilation; NAVA, neurally adjusted ventilatory assist; NIV, noninvasive ventilation; VILI, ventilator-induced lung injury.
The use of a global lung-protective ventilatory strategy, referring to low tidal volume and high levels of positive end-expiratory pressure (PEEP), in order to prevent ventilator-induced lung injury (VILI) improved survival in patients with acute respiratory distress syndrome (ARDS)4–8. In daily practice, the only way to assess the respiratory mechanics and the effects of MV on the lung itself are the ventilatory pressure, flow, and volume measured by the ventilator. However, relying on only these parameters during MV (plateau pressure level and tidal volume prescribed) may be misleading and may provide inaccurate assessment of the risk of VILI, since such variables do not accurately describe lung dynamics. Indeed, these recording variables reflect the respiratory system as a whole and do not take into account important pathophysiological features (e.g. chest wall compliance, intrinsic inspiratory/expiratory respiratory effort, heterogeneity of lung disease, etc.). Currently, new challenges are to optimize and customize MV by individualized monitoring at the bedside in order to avoid barotrauma, volotrauma, atelectrauma, and biotrauma9. To do so, transpulmonary pressure and capnography monitoring are helpful.
The driving pressure is a key variable for clinicians to optimize protective volume and inspiratory pressure in order to avoid lung stress and strain5,10. The driving pressure is the ratio of the tidal volume to the static respiratory system compliance (ΔP=VT/CRS); it is also equivalent to the plateau pressure minus the PEEP (ΔP = Pplat – PEEP). A recent study by Amato et al. on adult ARDS reported that among different ventilation variables, driving pressure was most strongly and independently associated with survival. Indeed, a decrease in driving pressure concomitant to a reduction in tidal volume or an increase in PEEP were associated with increased survival, while differences in tidal volume were not associated with different survival rates when the driving pressure was constant5. In ARDS, the proportion of lung available for ventilation is markedly decreased; therefore, the driving pressure (and consequently tidal volume) should be adapted to this reality rather than using only predicted body weight11. However, it is important to note that this approach should be adapted depending on the patient’s condition. In particular, the impact of a given driving pressure might not be similar in patients with low chest wall compliance12. It is the reason why there is an increasing interest in the monitoring of transpulmonary pressure to guide ventilatory assistance adjustments.
The transpulmonary pressure, defined by the difference between the airway pressure and pleural pressure, should be considered as the lung-distending pressure. This pressure measurement is closely correlated with lung strain and risk of VILI13. Esophageal pressure is a good surrogate for pleural pressure and its measurement is valuable to the assessment of lung strain in mechanically ventilated patients. Despite controversies regarding the interpretation of absolute values of esophageal pressure, a recent paper reviewed the usefulness of this tool in ventilation management14. Measurement of esophageal pressure is the only way to distinguish the effect of pressure on the lung and the chest wall. When a given amount of pressure is delivered, it is of great importance in some situations to better know which percentage is distending the lung (potentially harmful to the lungs) and which amount is distending the chest wall. As an example in ARDS, at the end of expiration, transpulmonary pressure can be negative (when pleural pressure exceeds end-expiratory airway pressure) and induces collapse of the alveoli. This may expose these parts of the lungs to being repeatedly reopened and recollapsed at each breath. To protect the lung from VILI, one would like to find a balance between protecting aerated units from over-distension and recruiting unstable units, thereby reducing tissue damage associated with their cyclic recruitment/derecruitment. The titration of PEEP based on esophageal pressure measurement15–17 has been proposed in patients with ARDS. Talmor et al. showed that oxygenation and lung compliance were significantly improved in patients managed by a ventilatory strategy including esophageal pressure measurement12. This recent interest in transplumonary pressure has contributed to the development of such monitoring in several ventilators (Avea ventilator-CareFusion® and G5-Hamilton Medical®, for example). An example of clinical information given by esophageal pressure monitoring is given in Figure 2. Unfortunately, such ventilators are not available in all units while no dedicated monitor is able to provide this measurement. We believe that the use of transpulmonary pressure has to be developed and, nowadays, we include this monitoring in our clinical practice in the management of difficult-to-ventilate children with low lung and chest wall (and/or low abdominal) compliances. However, more research in this field is needed to validate the best strategy to quantify esophageal pressure in children and to confirm its utility in ventilation titration. In particular, the impact of mediastinum weight is taken into account by some authors in adult studies, but it has not been examined in pediatric patients. Beyond the estimation of absolute pleural pressure, we also use esophageal pressure monitoring to assess the work of breathing in invasive ventilation and NIV (see below)18–20.
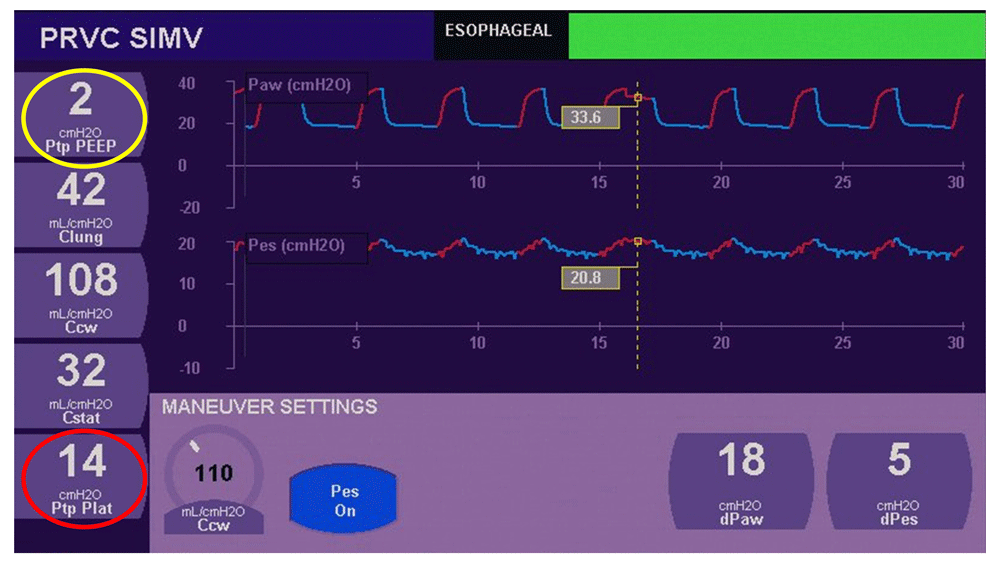
(1) Transpulmonary pressure (TPP = plateau pressure – esophageal pressure: to calculate TPP, esophageal pressure is used as a surrogate of intrapleural pressure) is 14 cmH2O at the end of insufflation (red circle). This means that despite a positive plateau pressure of 34 cmH2O in this obese child, lung parenchyma does not experience much distension (14 cmH2O) at the end of insufflation. (2) Positive end expiration pressure (PEEP) set on the ventilator is 2 cmH2O above esophageal pressure (yellow circle). This means that there is minimal risk of lung collapse at the end of expiration. Figure adapted from RM DiBlasi database with permission.
Volumetric capnography (Vcap) is also a novel tool which allows the measurement of physiological and alveolar dead space at the bedside21–23. In this technique, expired CO2 is plotted against the tidal volume for each breath. Vcap analysis gives a global index of ventilation/perfusion (V/Q) mismatch, containing shunt and indices of lung efficiency (physiological and alveolar dead space). Vcap can help to set PEEP to obtain the lowest physiological and alveolar dead space, the lowest arterial to end-tidal CO2 gradient (PaCO2–ETCO2 gradient), and the optimal alveolar plateau slope (SIII) that reflect V/Q heterogeneity24–27. We believe that Vcap will help clinicians to set PEEP routinely in the near future. We already use it to better predict PaCO2 in mechanically ventilated children23, and CO2 measurement obtained by Vcap is already included in a closed loop system dedicated to MV management28.
Increasing evidence suggests that MV is associated with diaphragmatic dysfunction and atrophy, also known as ventilator-induced diaphragmatic dysfunction29–31. To limit such consequences on the diaphragm, specific efforts should be addressed to reduce the duration of MV and to optimize ventilator settings. Improving individualized MV at bedside to limit diaphragmatic weakness is a great challenge but is essential to successfully wean patients from MV and decrease poor outcomes30,32,33.
Monitoring of the electrical activity of the diaphragm (EAdi) provides new information to clinicians in order to assess diaphragm function and the impact of ventilation on the diaphragm muscle that can lead to rapidly progressive diaphragmatic weakness30,32. EAdi has been shown to reflect the patient ventilatory drive, and it is well correlated with work of breathing based on short-term physiological studies34,35. EAdi permits the detection of periods of blunted drive secondary to overassistance36, which likely favor the risk of diaphragm dysfunction. It therefore may be used as a tool to adjust ventilatory support37, to detect tonic activity of the diaphragm (which reflects the effort of the patient to increase the lung volume)38, and to assess patient–ventilator asynchrony39. When combined with pressure or volume delivered, EAdi measurements permit the assessment of diaphragm neuroventilatory (VT/EAdi) or neuromechanical (ΔP/EAdi) efficiency40. In the only pediatric study on this topic to date, Wolf et al. observed that the ability to generate a higher diaphragmatic activity for the same tidal volume in pressure support ventilation (PSV) was a predictor of successful extubation41.
As shown in Figure 3, both EAdi and esophageal pressure can provide similar clinical information regarding the patient’s work of breathing. Although these tools are correlated in most clinical situations, they can differ in patients with diaphragmatic dysfunction. It is therefore important to emphasize that EAdi represents respiratory drive and not diaphragmatic contractility.
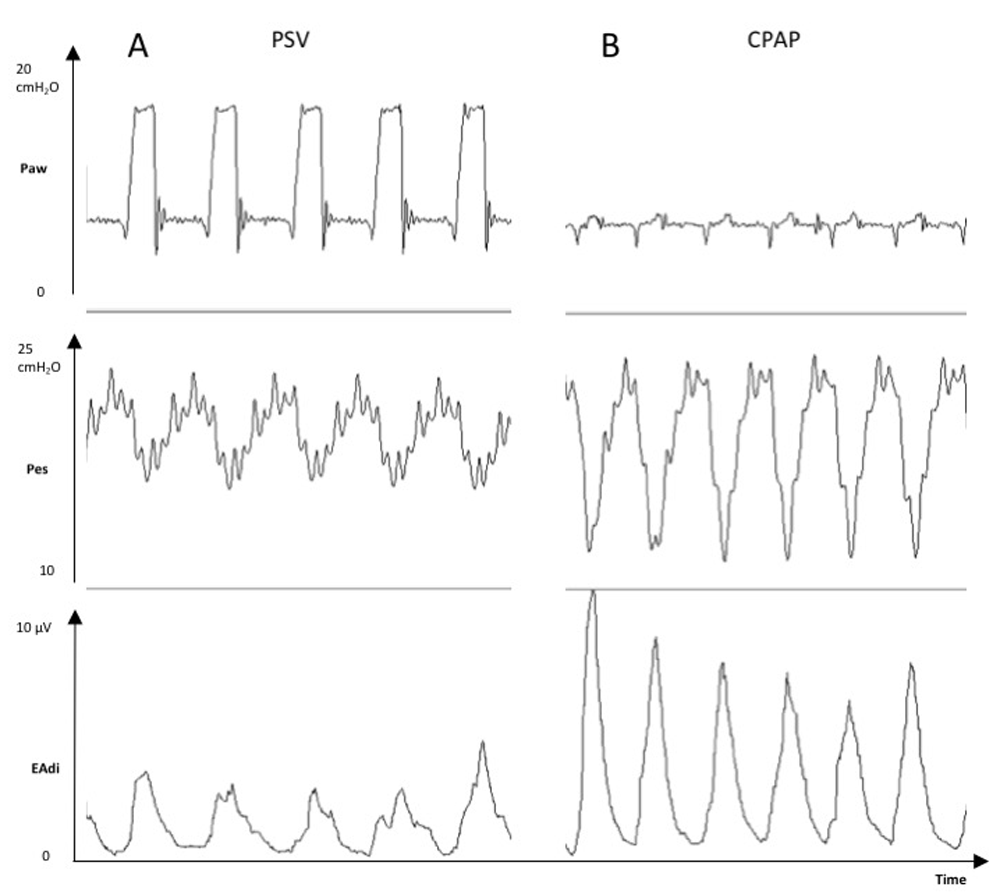
Tracings of a mechanically ventilated patient showing the increases in esophageal pressure swings (Pes, cmH2O) and electrical activity of the diaphragm (EAdi, μV) from a period with pressure support ventilation (PSV) +8 cmH2O and positive end-expiratory pressure +5 cmH2O (Panel A) to a period with continuous positive airway pressure (CPAP) +5 cmH2O only (Panel B).
Paw, mean airway opening pressure.
This technology requires a specific nasogastric catheter equipped with distal electrodes (NAVA catheter, Maquet Critical Care, Solna, Sweden) connected to a dedicated Servo-I ventilator (Maquet Critical Care, Solna, Sweden). The main clinical application of EAdi monitoring is the neurally adjusted ventilatory assist mode (NAVA), a mode of ventilation which uses the EAdi to trigger and cycle-off breathing efforts. The NAVA level and the EAdi determine the amount of ventilator assistance. NAVA has many advantages compared to conventional MV, including improved patient–ventilator synchrony39,42–45, the potential for a reduction in barotrauma (secondary to a decline of inspiratory pressure and tidal volume)23,39,42,44,46, a possible decrease in atelectrauma47, and, finally, improved diaphragmatic efficiency40. Moreover, NAVA improves unloading of the respiratory muscles and prevents the risk of over-assistance through downregulation of EAdi induced by increased assistance37. A recent randomized trial was conducted in children to test the clinical impact of NAVA48. The feasibility of NAVA in clinical practice was confirmed, and NAVA was associated with lower FiO2 requirements and lower inspiratory pressures. A trend for shorter duration of ventilation was observed, but it did not reach statistical significance. Nowadays, we use the NAVA mode routinely, in particular in difficult-to-wean children, in children who have undergone cardiac surgery, or any case in which the promotion of assisted ventilation and avoidance of diaphragm rest is important. EAdi is also routinely used to detect diaphragm contractility recovery in children with neuromuscular disease (e.g. botulism, Guillain-Barré syndrome, and cervical trauma).
Owing to MV’s potential complications, such as VILI49 and severe diaphragmatic atrophy30,32, it is imperative that it be discontinued as soon as the patient is capable of sustaining spontaneous breathing. On the other hand, premature extubation may also be problematic, as higher mortality rates have been reported in patients with extubation failure2,50. Consequently, when and how to perform MV weaning are key questions in critically ill patients. The identification of extubation readiness is usually based on clinical judgement, according to the respiratory, neurological, and hemodynamic status. However, this practice remains greatly subjective, while the timing of extubation is challenging. Therefore, efficient processes to safely reduce and remove ventilator support are necessary.
Clinical and research efforts have focused on early identification of weaning readiness. Some authors suggest the use of written protocols to assist clinicians in the management of weaning MV, but their usage in clinical practice remains limited for several reasons51: (1) providing and following protocols are time consuming, resulting in fluctuation in protocol implementation and compliance; (2) clinical instructions may not be explicit enough, resulting in variable interpretations of the protocol; and (3) protocols are generally specific to one organization, leading to a certain heterogeneity in clinical practice.
The development of the closed-loop system (CLS) (computerized protocol implementing its recommendations without caregiver intervention) has resolved some of these issues52. While optimizing ventilatory support on a continuous basis according to the patient’s respiratory condition, CLS offers consistent orders that constrain interpretation variations among caregivers, potentially resulting in a more efficient application of protocols. The use of CLS leads to a quicker adjustment of ventilator settings assessed by a reduction of time between the assessment of patient status and medical order, and medical order and clinical execution53.
Two CLSs are commercialized for respiratory weaning: SmartCare/PS® (Dräger Medical, Lubeck, Germany) and IntelliVent® (Hamilton Medical, Bonaduz, Switzerland). These systems automatically reduce the level of support when the patient’s respiratory rate, tidal volume, and end tidal CO2 (EtPCO2) are within acceptable ranges. In adults, these systems reduced the weaning time without increasing adverse events54. Currently, only two trials, one for each of these two technologies, have been conducted in children, and their findings regarding safety and duration of weaning process are encouraging28,53. A significant limitation of these systems remains the minimal weight/age required (15 kg for Smartcare/PS® and 7 kg with Intellivent®) and they cannot be used in case of significant leaks around the endotracheal tube. We believe that these automated systems will improve the management of MV and therefore the outcome of patients, allowing the customization of ventilator support according to each child’s condition. However, companies and researchers should now focus their efforts on algorithms adapted to our pediatric population.
During the weaning process, identifying whether or not patients will be able to breathe spontaneously after extubation is a significant challenge. The recent consensus conference on pediatric ARDS (PALICC) has addressed this question and recommended that spontaneous breathing trials (SBTs) or extubation readiness tests should be performed55.
Determining inclusion criteria for SBT initiation has been a difficult challenge because of the broad patient population, different modes of ventilation, and lack of consensus for acceptable SBT parameters. Another limitation is appropriate timing for starting SBT. For these reasons, some patients who qualify for SBTs may not be recognized, which may result in a prolonged ventilation course. Some institutions are now using electronic data pooled from ventilators and electronic medical records to develop explicit software rules and algorithms (decision support) to help identify patients who may be ready for SBT. Assuming a patient has met certain parameters for SBT criteria (EtCO2, SpO2, tidal volume, respiratory rate, inspiratory pressure, etc.), the electronic medical record can provide visual cues to help remind clinicians that their patient is ready for SBT.
In adults undergoing SBT, the use of an inspiratory pressure of 5 to 8 cmH2O is recommended56. In children, very few data exist regarding the optimal method to conduct a SBT. Interestingly, a physiologic study conducted by Khemani et al., comparing a SBT with a continuous positive airway pressure (CPAP) of 5 cmH2O versus pressure support of 10 cmH2O, concluded that pressure support significantly underestimates the potential for post extubation breathing efforts57. According to this recent study, we recommend performing a SBT in CPAP mode or with a T-tube. However, it should be noted that respiratory efforts observed during CPAP trial will be reflective of the efforts observed after extubation but will be larger than during SBT with PSV. Therefore, it is not surprising to observe increased efforts during CPAP, which should not lead to delay in extubation unless they appear to be objectively poorly tolerated.
During weaning, esophageal pressure measurement can be a useful tool to assess the work of breathing. A robust parameter which can be derived from esophageal pressure and transdiaphragmatic pressure, i.e. the difference between esophageal pressure and gastric pressure, is the pressure-time-product. This parameter was used as a tool to assess work of breathing and optimize ventilation support in children with different diseases18,20. Jubran et al. showed that esophageal pressure trend during a SBT provided an accurate prediction of weaning outcome58. Over the course of a SBT, esophageal pressure-time-product remained unchanged in successfully weaned patients. In contrast, weaning failure patients developed marked and progressive increase in esophageal pressure-time-product (up to 4-fold above the normal value) as a result of an increase in the mechanical load of the respiratory muscles58.
HFOV has been commonly used for decades in neonatal, pediatric, and adult populations58. Clinical trials have demonstrated that HFOV is associated with an oxygenation improvement in patients with acute lung injury or ARDS59–61. However, the clinical use of HFOV in this population has decreased. Recent studies demonstrated an association between early use of HFOV and worse outcome in terms of mortality in adult62 and pediatric populations63,64. However, several biases have been highlighted in the two pediatric studies regarding the methodology65–67. As suggested by Rettig et al., the mortality in patients with ARDS supported by HFOV may be linked to the disease category itself rather than the use of HFOV68. Given these limitations and with regard to our clinical experience, we consider, as supported by the PALICC, HFOV to still be a rescue therapy in some children with severe ARDS.
NIV is defined as the delivery of MV without an endotracheal tube or tracheostomy. NIV comprises both CPAP and bilevel positive airway pressure (BiPAP) ventilation. NIV is increasingly used in PICUs69,70. In the last decade, the potential indications for NIV in critically ill patients have grown considerably, and the performance of this mode of support has greatly improved. In children developing ARDS, NIV can be considered as a first line of treatment in milder disease55. Despite the lack of clear guidelines, this mode of support definitely has its place in the treatment of a wide range of pathologies in children, including pneumonia, upper airway obstruction, post-extubation respiratory failure, acute chest syndrome, and asthma70.
The use of NIV has recently evolved because of the emergence of high-flow nasal cannula (HFNC). This modality is now available from a number of manufacturers and has been widely adopted in pediatric practice. Different mechanisms have been hypothesized to account for the clinical benefits, including washout of the nasopharyngeal dead space, reduction of work of breathing, decrease in airway resistance, and improvement of pulmonary compliance71,72. HFNC has been able to provide a mean pharyngeal pressure of 4 cmH2O when used at a flow of 2 L/kg/minute73, but this effect is variable. In clinical use, HFNC allows improvement of comfort and tolerance to NIV and reduction of air leak, gastric distension, and skin injuries, especially in younger children. The literature is still poor to identify the specific population that would benefit from this technology18,74. The role of HFNC outside the PICU still needs to be investigated, and we currently restrict HFNC use in the PICU. More evidence is expected from several ongoing randomized controlled trials (TRAMONTANE study, NCT02457013; Hi-Flo study, NCT01498094; HHFNC study, NCT01662544). We believe that, within a few years, the role of HFNC will be better defined and potentially widened.
The optimal interface for NIV in children has recently been discussed as a key aspect in respiratory management75. A large variety of devices recently emerged, including nasal, oronasal, and total face masks and helmet. Because mask-fit pressure is spread over a larger surface beyond the nose area, total face masks appear to be more comfortable than oronasal masks76. This device was shown to be as efficient as oronasal mask in terms of breathing pattern, gas exchange, and outcome in adults77. The helmet is also increasingly used70 and should be considered as a feasible alternative for NIV in children, as suggested by the results of a recent randomized controlled trial comparing the use of a helmet and a face mask in children78. As for total face masks, preliminary data are pointing towards the helmet as an interface to increase comfort and decrease skin injury and air leaks79.
Finally, to improve NIV success, the achievement of an adequate patient–ventilator synchrony is crucial19. Although the performance of ventilators has improved within the last few years, patient–ventilator asynchrony in NIV remains a significant issue. As with invasive ventilation, tools to improve patient–ventilator synchrony during NIV have been recently investigated. EAdi monitoring and noninvasive NAVA are feasible and well tolerated in PICU patients with patient–ventilator synchrony improvement80,81. Monitoring esogastric pressure offers another way to improve patient–ventilator interaction during NIV. In infants82 and children19, esophageal pressure measurement has been shown to be a valuable tool to assess patient–ventilator interaction and to optimize ventilatory settings (Figure 1).
There have been major advances in the management of mechanically ventilating children over the last 3 years. The implementation of this new knowledge in usual practice is a challenge, as advances occur not only in the respiratory field but also in many fields that pediatric intensivists must digest. In such a situation, companies that design medical devices including ventilators and respiratory monitoring platforms play a key role in the application of knowledge. The creation of a ventilation consortium that includes companies, caregivers, researchers, and stakeholders could be a solution to promote knowledge implementation.
Philippe Jouvet and Guillaume Emeriaud have a salary from the Public Research Agency of Quebec (FRQS). Philippe Jouvet is a consultant for Sage Therapeutics Inc. and has been invited to a congress by Covidien, Medunik Inc., and Air Liquide Santé. Medical devices are lent to Philippe Jouvet by Hamilton Medical, Philips Medical, and Maquet. The other authors have no competing interests to disclose.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 17 Mar 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)