Keywords
stem cell therapy, skin cells, osteogenesis
stem cell therapy, skin cells, osteogenesis
Finding appropriate therapeutic cells for bone regeneration has been a challenge for decades. Recently, stem cells from the skin, a potentially large cell source with easy access, have caught the attention of clinicians and scientists. More and more evidence indicates that skin stem cells are a potential cell source for bone regeneration. For example, heterozygous inactivating mutations of GNAS (encoding guanine nucleotide-binding G protein alpha subunit) cause diseases, including progressive osseous heteroplasia, Albright hereditary osteodystrophy, pseudohypoparathyroidism, and osteoma cutis1–4. These disorders have the common features of superficial ossification, starting with cutaneous ossification, with some involving subcutaneous and deeper tissues and some restricted to the skin. Multipotent progenitor cells and bone morphogenetic proteins (BMPs) were reported to be responsible for ectopic ossification5,6.
Despite a decade of investigations using skin stem cells for regenerative medicine, most literature concerns their application in skin tissue engineering7 and nerve regeneration8, which was well covered by a recent review article9. However, few review articles are available on skin cell-based osteogenesis. This review first summarizes the latest findings on stem cells or progenitors in skin and their niches and then discusses the strategies of skin cell-based osteogenesis (Figure 1). We hope this article elucidates this topic and generates new ideas for future studies.
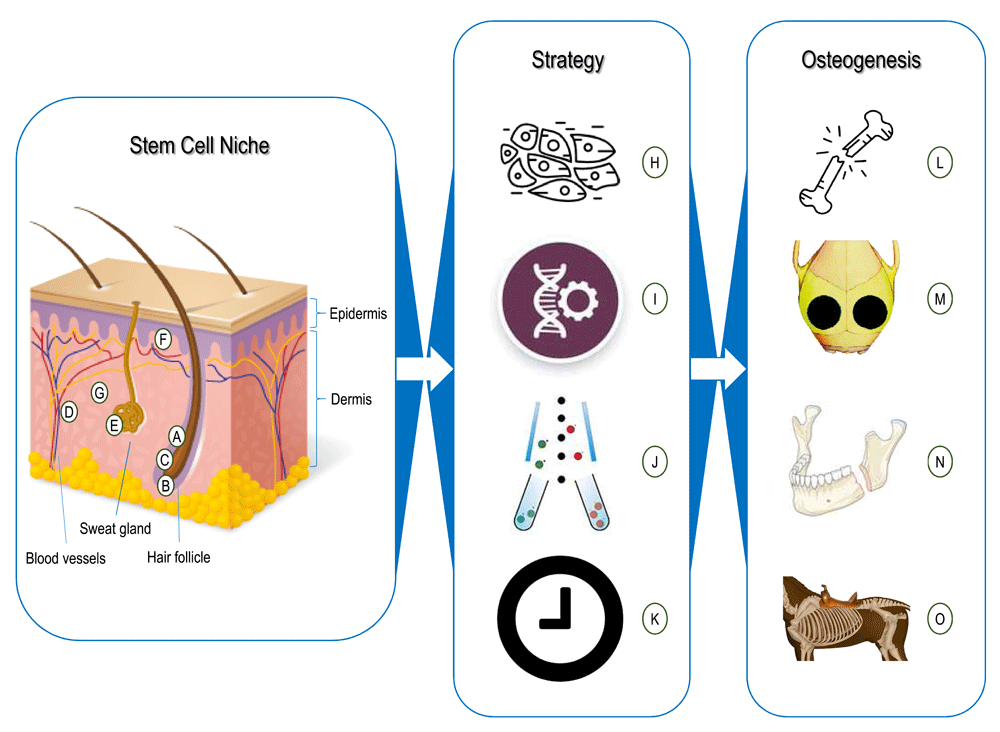
(A–G) Stem cells and niches found in skin. (A) Hair follicle bulge-derived stem cells11,12,15. (B) Hair follicle papilla-derived stem cells18,22–24. (C) Hair sheath-derived stem cells16,22. (D) Pericytes10,51. (E) Sweat gland-derived stem cells25,26. (F) Interfollicle epidermis-derived stem cells13,14. (G) Stem cells from dermal niches that are not fully characterized27–34,50,52,53. (H–K) Strategies for using skin cells. (H) Total skin fibroblasts35,36. (I) Genetic modification38–48. (J) Cell sorting33,50–53. (K) Cell reprogramming56–58,65. (L–O) Skin cells’ osteogenesis. (L) Limb bone defect regeneration35,41,42. (M) Cranial bone defect regeneration38,43,44,53. (N) Mandibular bone defect regeneration40,48. (O) Rib bone defect regeneration45.
Besides the primary structure of the epidermis, dermis, and subcutaneous tissue, there are hair follicles, vessels, capillaries, neurons, sweat glands, sebaceous glands, lymphatic capillaries, and erector pili muscles in skin, implying that there could be numerous niches for stem cells and progenitors in this tissue (Table 1). Evidence also indicates that stem cells in skin, so-called pericytes, might be of perivascular origin10.
| Location | Niche | Culture | Name | Markers | Differentiation potential | References |
|---|---|---|---|---|---|---|
| Epidermis | Interfollicle epidermis | Adherence | Epidermal stem cells | α6 integrin, β1 integrins, CD133, CD90, and keratin 15 | Keratinocytes | 13,14 |
| Hair follicle and appendages | Hair follicle bulge | Adherence | Keratinocyte stem cells/ epidermal neural crest stem cells | Keratin 15, keratin 19, β1 integrins, CD200, PHLDA1, follistatin, frizzled homolog 1, CD24lo, CD34lo, CD71lo, and CD146lo | Keratinocytes, all major neural crest lineages, including neurons, Schwann cells, myofibroblasts, melanocytes, and bone/ cartilage cells | 11,12,14,15 |
| Hair follicle sheath | Floating spheres | Dermal sheath cells | Nestin, fibronectin, CD34, and keratin 15(−) | Adipogenic and osteogenic lineages | 16,22 | |
| Hair follicle papillae | Floating spheres | Skin-derived precursor cells | βIII-tubulin, p75NTR, NF-M; CNPase, GFAP, and S100β | Adipogenic, osteogenic, chondrogenic, and myogenic lineages, neurons, glia, and Schwann cells | 18–23 | |
| Sweat gland | Adherence | Sweat gland stroma- derived stem cells | α6 integrin and nestin | Adipogenic, chondrogenic, and osteogenic lineages | 25,26 | |
| Dermis | Perivascular | Adherence | Pericytes | CD146, NG2, CD31(−), CD34(−), CD144(−), and VWF(−) | Adipogenic, chondrogenic, myogenic, and osteogenic lineages | 10,51 |
| Undefined niches of dermis | Adherence | Dermal stem cells/dermis- derived stromal cells | CD13, CD29, CD44, CD49d, CD71, CD73, CD90, CD105, CD166, SSEA4, vimentin, CD14(−), CD31(−), CD34(−), CD45(−), CD106(−), CD133(−), SSEA3(−), and nestin(−) | Adipogenic, chondrogenic, myogenic, and osteogenic lineages | 27–34,50,52,53 |
Epidermal stem cells are found in both hair follicle bulge11,12 and interfollicular epidermis13,14. They are also viewed as keratinocyte stem cells because they generate cells that produce keratin11,14. Recent reports indicate that human epidermal stem cells are able to create all major neural crest derivatives containing neurons, Schwann cells, myofibroblasts, melanocytes, and bone/cartilage cells15,16. Despite the investigation of many stem cell markers, such as α6 integrin 5-bromo-2-deoxyuridine, β1 integrins, CD133, CD200, CD90, keratin 15, delta 1, and p6317, the molecular signature of epidermal stem cells remains undetermined.
Hair follicles have long been considered an important niche for stem cells because of the versatility in regeneration of hair and epidermis and wound repair. For example, skin-derived precursors (SKPs) from both murine and human origins residing in the papillae of hair follicles18 can differentiate into neuron, glia, smooth muscle, and adipose cells19,20. As non-adherent cells, the SKPs are cultured as floating spheres with a neural crest origin21. Although lineage differentiation crosses both ectoderm and mesoderm18,20, their potential for osteogenesis has seldom been tested, although a cell subpopulation characterized from hair follicle dermal papilla and dermal sheath of both rats and humans has the capacity for adipogenesis, myogenesis, chondrogenesis, and osteogenesis22–24. In addition, since keratinocytes can be generated from the hair follicle bulge, the hair follicle is an important niche for epidermal stem cells11,12. These findings indicate that the hair follicle is one of the most important niches in skin with stem cells and progenitors generating mesenchymal lineages. Recent studies indicate that sweat glands, a skin appendage, are also characterized as a niche for stem cells which can be isolated and induced into three mesodermal lineages25,26.
Dermis constitutes the majority of skin in both thickness and cell number. Dermal fibroblasts, the principal cells in dermis, have long been considered terminally differentiated cells and served as a negative control of mesenchymal stem cells (MSCs). When preserved in saline at 4°C for 6 days before digesting, non-hair follicle human dermis has been successfully proven to be an MSC source, indicative of a potential niche for stem cells27. This finding is supported by another report, in which clonal analysis of a single dermal fibroblast isolated from human foreskin exhibited tripotent, bipotent, and unipotent ability28, indicating multiple differentiation potential in dermal fibroblasts. Increasing evidence also demonstrates that these cells are positive for surface markers CD29, CD44, CD73, CD90, CD105, and CD166, indicating their MSC nature, and negative for CD14, CD31, CD34, CD45, and CD133, indicating non-hematopoietic lineage29–34.
Fibroblasts from rabbit skin were osteoinduced followed by seeding on porous titanium pylon; this construct exhibited enhanced osseointegrative properties compared with unseeded pylon in both in vitro and in vivo studies35. This study and others36 suggest the possibility of using skin fibroblasts for osteogenesis, although an early report showed the inhibition of rat skin fibroblasts on mineralization of bone marrow MSCs37. Unfortunately, owing to the low osteogenic potential of total skin fibroblasts with mixed cell populations, this kind of trial is far from successful. Therefore, it is critical to isolate skin cells with a preference for differentiation toward osteogenesis.
Using modification of genes to increase the expression of specific osteogenesis-related genes, skin fibroblasts, acting as “protein secretors” without differentiating by themselves or having the paracrine/exosomal effects that are found in MSCs, were promoted for bone tissue engineering and regeneration38–41. These genes of interest include BMP-241–45, BMP-442, BMP-738,42, Runx2 (runt-related transcription factor 2)39,43,46,47, and LMP-3 (lim mineralization protein-3)40,48. In in vivo studies using skin fibroblasts, both ectopic osteogenesis and orthotopic bone regeneration are achieved through gene therapy42,44 from small animals like mice44, rats38,42,48, and rabbits41 to large animals like equines45. A study comparing different genes of interest for modification efficiency of skin fibroblasts determined that BMP-2 is more powerful than Runx243 and that the mineralization ability of Runx2-modified skin fibroblasts is scaffold-dependent39. Gene therapy is a promising method with a prominent effect; however, the safety of viral genetic modification needs further characterization49.
Mixed populations isolated from total skin make cell therapy strategies for osteogenesis unsuccessful. Consequently, there are increasing efforts in sorting cells from skin to get target subpopulations. For example, type IV collagen-coated dishes have been used to attract CD29(+) human dermal stem cells via adherence, which exhibited higher osteogenic, adipogenic, and chondrogenic capacity compared with unsorted cells33. CD271(+) and CD146(+) cells isolated from human skin and CD73(−)CD105(+) cells isolated from mouse skin by immunosorting also showed elevated multi-differentiation potential50–52. Interestingly, subpopulations sorted by other markers from human skin, such as CD73, stage-specific embryonic antigen-4 (SSEA-4), and BmprIB, show relatively restricted differentiation potential. For instance, BmprIB(+) cells can generate only an osteogenic lineage50,53, indicating that these subpopulations can be applied as therapeutic cells for osteogenesis because of their established lineage preference. However, concern due to low harvest rate resulting from cell sorting still exists50,51,53.
Characterized by unlimited proliferation and differentiation potential like embryonic stem cells54,55, induced pluripotent stem cells (iPSCs) can be used in numerous stem cell therapies. As skin fibroblasts are the most abundant and easily accessed cells, they are commonly chosen as the parent cells of iPSCs. It has been well characterized that iPSC-derived osteoblasts can form osteoid both in vitro and in vivo56–58. A recent study revealed that bone defect repair is also achieved by human iPSCs in a radial defect model of immune-deficient mice59. Furthermore, the involvement and mechanism of microRNAs in the regulation of mouse iPSCs during osteogenic differentiation have been preliminarily investigated60.
In past decades, investigations using skin cells for osteogenesis have achieved significant progress. Many niches for stem cells in skin have been revealed and preliminarily characterized. Also, skin cells, enriched or not enriched, modified or not modified, are used for osteogenesis in vitro and in vivo and have achieved success in limb, cranial, mandibular, and rib bone defect regeneration (Figure 1). However, some key problems remain unsolved. For example, since the niche for stem cells in dermis is not completely characterized, the efficiency of enriching stem cells or progenitors from skin is still restricted. For cell modification strategies, like gene therapy and cell reprogramming, the efficacy might be readily apparent, but the safety needs more in-depth research.
Recent developments in epigenetic conversion may shed some light on cell reprogramming. Unlike in iPSCs, epigenetic conversion does not completely reverse cells to the pluripotent stem cell stage61–64. This approach may avoid undesired side effects such as teratoma, which often occurs in the application of iPSCs and embryonic stem cells. Epigenetic conversion has achieved progress in directing fibroblasts from human skin and mouse embryos into cardiomyocytes, neuronal cells, and insulin-secreting cells with a mature phenotype61,63,64. Although not much is known about converting skin fibroblasts into osteoblasts, there is a report of converting non-osteogenic cells into osteoblasts by epigenetic stimulation of BMP-2 expression65. By transient use of platelet-derived growth factor-AB and 5-azacytidine, mature bone and fat cells can also be converted into multipotent stem cells62. Thus, although there are no studies characterizing the cells converted for bone regeneration, the most common candidate for epigenetic conversion, skin cells, may play a significant role in this strategy.
Taken together, two of these strategies are promising. One strategy is the enrichment of stem cells and progenitors from different skin niches. By improving the current low-efficiency cell isolation, a mass of therapeutic cells can be gathered from skin for better bone tissue engineering and regeneration. The other strategy is based on the easy access and abundant amount of skin fibroblasts. Via modification of the cell, either through iPSCs or the recent concept of epigenetic conversion, a differentiation-specific cell population can be manipulated and gathered. In that case, therapeutic cells for osteogenesis can be harvested on a large scale, making both the autologous and allogeneic approaches possible.
This work was supported by research grants from the Musculoskeletal Transplant Foundation and the National Institutes of Health (1R03AR062763-01A1 and 1R01AR067747-01A1).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 17 Mar 17 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)