Keywords
cell signaling, protein oxidation, reactive oxygen species, redox signaling,
cell signaling, protein oxidation, reactive oxygen species, redox signaling,
Reactive oxygen species (ROS) have been shown to play important roles in cell signaling (Finkel, 2011; Suzuki et al., 1997). In particular, the roles of ROS in cell growth signaling have been well documented (Rao & Berk, 1992; Sundaresan et al., 1995). For the mechanism of ROS signaling, the receptor activation producing ROS via NAD(P)H oxidase is a widely accepted concept (Griendling et al., 1994). However, molecular targeting mechanisms for ROS in cell signaling have been unclear. ROS targeting protein cysteine thiols has been the most popular proposed mechanism (D’Autreaux & Toledano, 2007; Forman et al., 2010; Moran et al., 2001; Rhee et al., 2000; Sen, 2000; Truong & Carroll, 2012; Veal et al., 2007), yet the occurrence of thiol oxidation requires levels of ROS that are much higher than what is expected to occur during cell signaling (Burgoyne et al., 2007).
Our laboratory has proposed that ligand/receptor-mediated cell signaling involves protein carbonylation (Wong et al., 2008; Wong et al., 2010), which occurs on four susceptible amino acid residues: proline, arginine, lysine, and threonine (Amici et al., 1989; Berlett & Stadtman, 1997). Notably, in cultured cells, hydrogen peroxide (H2O2) as low as 0.5 µM was found to promote protein carbonylation (Wong et al., 2008).
More recently, we identified proteins that are carbonylated in response to the platelet-derived growth factor (PDGF) stimulation. Among them, peroxiredoxin-6 (Prx6) was found to be carbonylated in response to a 10-min treatment of human pulmonary artery smooth muscle cells with PDGF (Wong et al., 2013). Peroxiredoxins have been shown to regulate cell signaling (Woo et al., 2010). The present study tested whether this signaling mechanism also promotes sulfhydryl oxidation within the Prx6 molecule.
HPASMCs (ScienCell Research Laboratories, Carlsbad, CA, USA) were serum-starved overnight and treated with recombinant human PDGF-BB or H2O2 for 10, 15 or 30 min. Protein thiol states were monitored using SulfoBiotics Protein Redox State Monitoring Kit Plus (Dojindo Molecular Technologies, Rockville, MD, USA) in accordance with the manufacturer’s instructions. Briefly, cells were washed, proteins precipitated with trichloroacetic acid and “Protein-SHifters” were added to each sample. Samples were then loaded onto a sodium dodecyl sulfate polyacrylamide gel and electrophoresed. The gel was exposed to UV light to cut the “Protein-SHifters.” The resultant non-reducing SDS polyacrylamide gel was electroblotted to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA, USA). The membrane was blocked with 5% milk for 30 min at room temperature and incubated with the anti-Prx6 antibody produced in rabbit (Sigma-Aldrich Chemical Company, St. Louis, MO, USA; Catalogue no. P0058; 1:1,000 dilution) at 4°C overnight. The membrane was then washed three times and incubated with goat anti-rabbit IgG-horseradish peroxidase conjugate (Bio-Rad; Catalogue no. 1706515; 1:3,000 dilution) for 45 min at room temperature. After washing three times, signals were obtained using an Enhanced Chemiluminescence System (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA).
The technology developed for SulfoBiotics Protein Redox State Monitoring Kit Plus, by Dojindo Molecular Technologies adds a 15 kDa Protein-SHifter on free sulfhydryl groups, allowing the visualization of the thiol status of a given protein by coupling with immunoblotting. The human Prx6 molecule with a molecular weight of 25 kDa has two cysteine residues. Our results indicated that untreated human pulmonary artery smooth muscle cells predominantly contain the 55 kDa species, consistent with the Prx6 molecule, which has two Protein-SHifters incorporated, indicating that both cysteine residues occur in the reduced form in the cells (Figure 1A, lane 1). Treatment of cells with PDGF (10 ng/ml) for 10 min, which promoted protein carbonylation of Prx6 (Wong et al., 2013), did not alter the thiol state of Prx6 (Figure 1A, lane 1 and lane 2). The PDGF treatment for 30 min did not alter the thiol state of Prx6 either (Figure 1A, lane 1 and lane 3). By contrast, treatment of H2O2 at a high concentration (1 mM) eliminated the 55 kDa band and generated a 40 kDa band that is consistent with one sulfhydryl group being oxidized (Figure 1A, lane 4). These results were reproduced at least five times. Dataset 1 (Suzuki et al., 2017) contains the uncropped version of Figure 1A and the uncropped repeats. The bar graph shows the data from five separate experiments with five separate cell treatments. Control experiments were performed to ensure that PDGF stimulated protein phosphorylation as well as carbonylation.
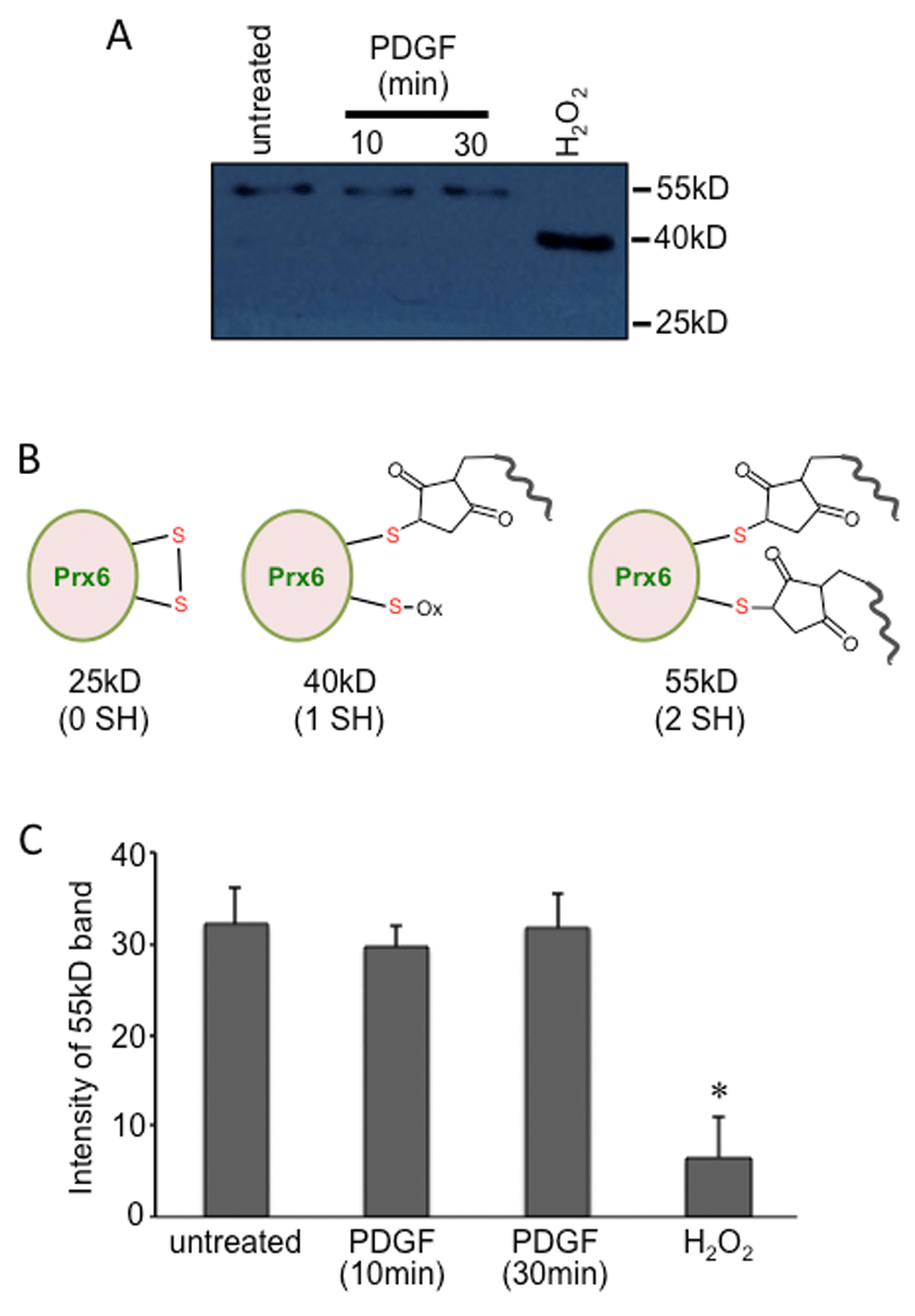
Human pulmonary artery smooth muscle cells were treated with PDGF (10 ng/ml) for 10 or 30 min as described in Wong et al. (2013), or with H2O2 (1 mM) for 15 min. Cellular proteins were precipitated with trichloroacetic acid and lysate samples were prepared in accordance with the manufacturer’s instructions for SulfoBiotics Protein Redox State Monitoring Kit Plus (Dojindo). The Protein-SHifter Plus that covalently binds to reduced protein thiols was added and the samples were subjected to electrophoresis through a 12% polyacrylamide gel. Each Protein SHifter Plus causes ~15 kDa shift of the protein bands. After electrophoresis, the gel was exposed to UV irradiation to excise the Protein-SHifter Plus moiety, and then subjected to electrotransfer to a nitrocellulose membrane and Western blotting with the Prx6 antibody. (A) Representative Western blotting image of six experiments. (B) Diagram of the native 25 kDa Prx6 molecule, the 40 kDa Prx6 molecule with one Protein-SHifter attached, and the 55 kDa Prx6 molecules with two Protein-SHifters attached. (C) The bar graph represents means (± SEM) of the intensity of the 55 kDa band (N = 5). The symbol (*) denotes that the value is significantly different from all other values.
Unlike protein carbonylation of Prx6, which is promoted in response to PDGF-treatment of human pulmonary artery smooth muscle cells (Wong et al., 2013), PDGF stimulation of cells does not cause the oxidation of two cysteine residues within the human Prx6 molecule. By contrast, cysteine oxidation within the Prx6 molecule can be promoted by treating cells with mM concentrations of H2O2 that are not likely to be generated in ligand/receptor-mediated cell signaling. We conclude that protein carbonylation, but not sulfhydryl oxidation, is a likely ROS-targeting mechanism for growth factor stimulation and cell signaling.
Protein carbonylation is promoted by metal-catalyzed generation of hydroxyl radicals, which are known to promote oxidation indiscriminately. However, the caged and site-directed production of hydroxyl radicals via metals could confer specificity (Stadtman & Berlett, 1991; Wong et al., 2010).
Dataset 1. The uncropped version of Figure 1A and the uncropped repeats.
DOI, 10.5256/f1000research.11296.d157362 (Suzuki et al., 2017)
YJS conceived the study and designed the experiments. CC, FA, LM, VR, and YJS carried out the research. YJS prepared the first draft of the manuscript. All authors were involved in the revision of the draft manuscript and have agreed to the final content.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute and National Institute of Aging (Grants R01 HL72844 and R03 AG047824) to YJS. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Angiogenesis, ROS signaling, NO biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 10 Apr 17 |
read | read | read |
Click here to access the data.
Spreadsheet data files may not format correctly if your computer is using different default delimiters (symbols used to separate values into separate cells) - a spreadsheet created in one region is sometimes misinterpreted by computers in other regions. You can change the regional settings on your computer so that the spreadsheet can be interpreted correctly.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)