Keywords
Wilms’ tumor, inflammatory myofibroblastic tumors, IMTs, neoplasms of uncertain behavior, ileal conglomerate, ileus,
Wilms’ tumor, inflammatory myofibroblastic tumors, IMTs, neoplasms of uncertain behavior, ileal conglomerate, ileus,
The author information of Rikke Raagaard Soerensen is changed to Department of Pathology, Herlev Hospital, University of Copenhagen, Herlev, Copenhagen, Denmark instead of Department of Pathology, Hvidovre Hospital, University of Copenhagen, Hvidovre, Copenhagen, Denmark. Rikke Raagaard Soerensen was during this work employed at Herlev Hospital.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Inflammatory myofibroblastic tumors (IMTs) are rare mesenchymal neoplasms composed of proliferating myofibroblastic spindle cells, and an accompanying inflammatory - usually chronic – infiltrate1. The etiology of IMTs is unknown, but an association with Wilms’ tumor, the most common primary renal malignancy in children, has been suggested2,3. Furthermore, theories suggesting a link to infectious agents, tumor associated factors, and cytokines have been proposed4. IMTs are most commonly seen in children and young adults1. IMTs were initially considered a pulmonary tumor2, but the lesions have subsequently been reported in various extrapulmonary organs including the mesentery and gastrointestinal tract4.
This report presents a case of an IMT in the terminal ileum in a female adult, treated for Wilms’ tumor in childhood. The tumor caused a conglomerate of small bowel and mimicked Meckel’s diverticulitis, which to our knowledge has not previously been described. We reported this case according to the CARE guidelines5.
A 44-year-old woman was admitted to the hospital due to intermittent severe abdominal pain, during which a visible bulge appeared in the right lower abdominal quadrant. Furthermore, the patient had experienced loose stools over a two year period. The initial blood tests were inconspicuous, with the exception of a slight neutrophilia. The past medical history included treatment for a Wilms’ tumor at the age of one with a right-sided nephrectomy and subsequent radiochemotherapy.
Ileus was initially suspected, and an abdominal CT scan was performed, showing a 3 × 5 × 4 cm mass in the small intestine. The imaging report described, that the lesion was composed of a solid and a necrotic portion plus an air-filled space (Figure 1), which lead to the tentative diagnosis of an underlying Meckel’s diverticulitis. A diagnostic laparoscopy was initiated, but converted to an open ileocecal resection with a primary anastomosis, as a tumor in the terminal ileum was discovered. There was no sign of intestinal perforation.
Grossly, the specimen consisted of a cecal pole that included the appendix and the distal 40 cm of the terminal ileum bound together in a conglomerate (Figure 2). 10 cm from the proximal resection margin a diverticulum-like pouch arising from the ileal wall was identified. The lesion contained a solid tumor of firm consistency, that measured 3 (depth) × 5 (width) cm. The cut surface of the tumor elicited heterogeneity with white/grayish areas and focal haemorrhage. The tumor was totally embedded.
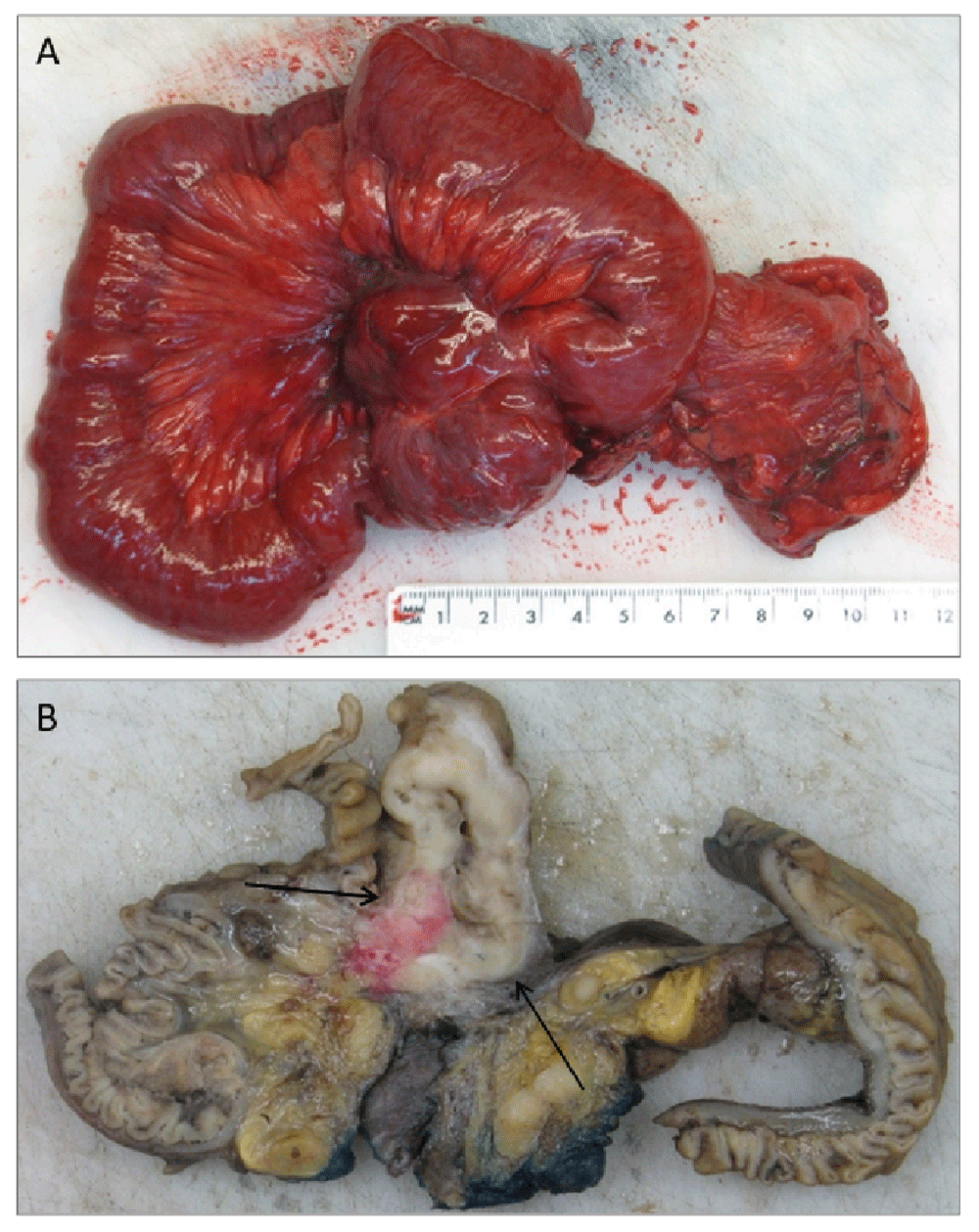
The gross appearance of the tumor before (A) and after (B) formalin fixation. The tumor (indicated by arrows) infiltrated the wall of the small intestine and the adjacent mesoileum (B), creating a conglomerate of dilated small bowel (A).
Microscopy revealed a mesenchymal tumor infiltrating the ileal wall, the submucosa and adjacent mesentery. No true diverticulum was found. The tumor was composed of a dense proliferation of spindle-shaped cells primarily arranged in a fascicular growth pattern, focally also exhibiting a storiform architecture, admixed with a marked chronic inflammatory infiltrate and discrete areas of neutrophilic granulocytes (Figure 3). Immunohistochemically, the tumor cells were positive for actin, vimentin, desmin, and h-caldesmon, focally positive for factor 13A and CD68, and had a negative reaction for ALK1, CD34, CD117, DOG1, S100, AE1/AE3, CK18, CK7, CK19, beta-catenin and MDM2. Ki-67 staining showed a low proliferative rate. Although h-caldesmon reactivity was considered atypical for the tumor, the pathology report characterized the tumor as an IMT based on morphology and additional staining properties.
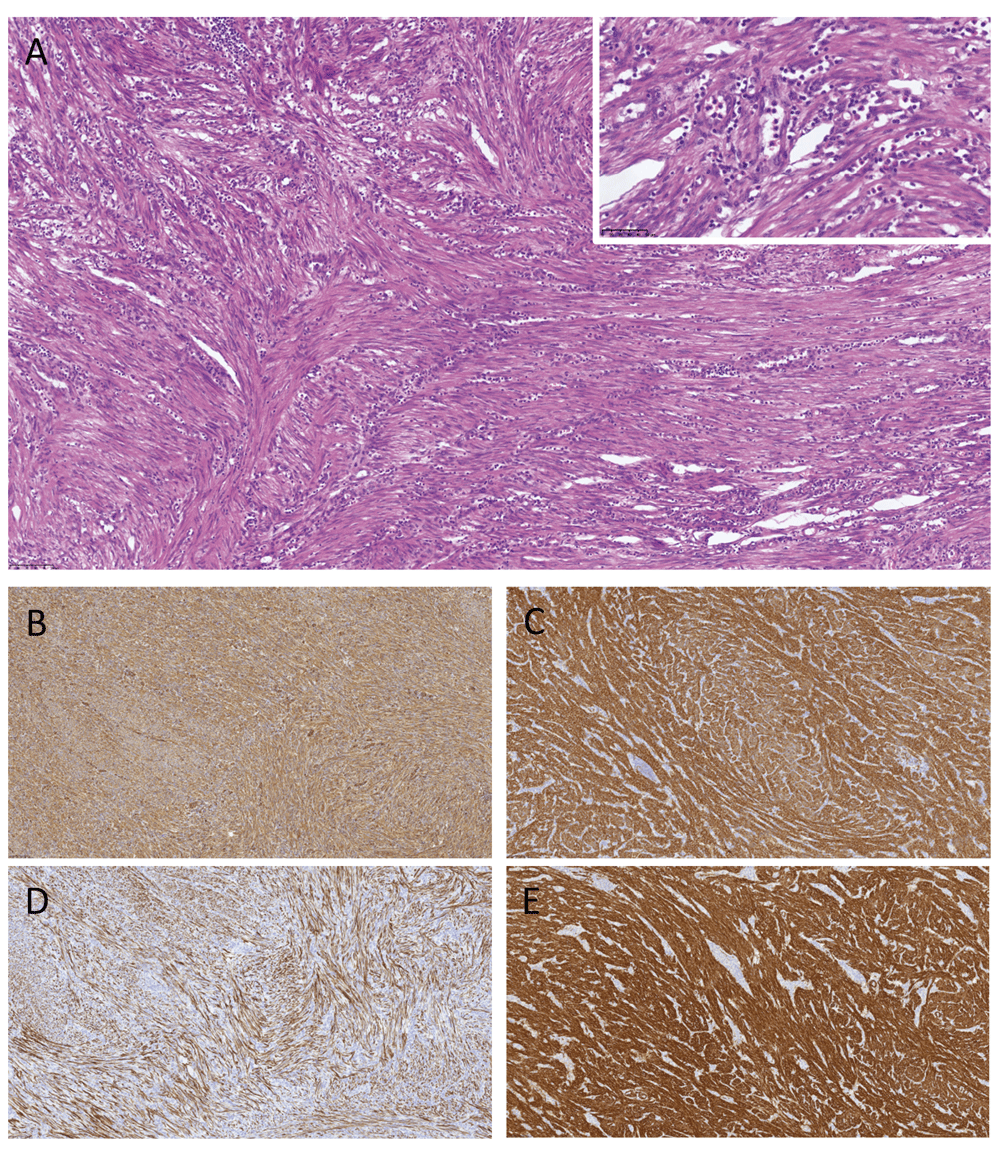
The tumor mass was built from bundles of spindled myofibroblastic cells with a low proliferative rate arranged in a crossing manner, accompanied by a pronounced lymphocytic infiltrate. Focally, infiltration with polynuclear granulocytes was observed (A, shown in the insert). Immunohistochemically, the tumor cells were positive for vimentin (B), actin (C), desmin (D) and h-caldesmon (E).
Postoperatively, the patient suffered several complications including an intraabdominal abscess caused by a small anastomotic leakage. Subsequent percutaneous drainage of the abscess led to a phlegmone involving the abdominal wall, complicated by the development of a transcutaneous fistula with connection to the peritoneum. Six months after surgery the patient was still having symptoms from the transcutaneous fistula but was otherwise well. She was advised to have a control abdominal magnetic resonance imaging (MRI) after one year.
We reported a case of an adult female patient presenting with an IMT in the terminal ileum causing a conglomerate of small intestinal loops leading to obstructive ileus. A previous study has reported that only 1.2% of IMTs arise from the small intestine3. It has been described that IMTs in the small bowel can cause intestinal obstruction due to intussusception1. However, in our case a conglomerate of small bowel was the cause of ileus, which to our knowledge has not been presented previously in the literature. In general, patients suffering from IMTs may clinically present with an abdominal mass or non-specific symptoms including abdominal pain6. In approximately 15–30% of cases the patients present with a constitutional syndrome of fever, weight loss, malaise and a variety of laboratory abnormalities such as anemia, thrombocytosis, leukocytosis, polyclonal hyperglobulinemia, or elevated erythrocyte sedimentation rate3. In this case the subject only presented with a few of these features.
Macroscopically, IMTs in the gastrointestinal tract are most often characterized as solid, sessile, and solitary lesions, although cases with multiple lesions have been described4. In this case the tumor mimicked a diverticulum and based on the clinical presentation, numerous differential diagnoses were possible including Meckel’s diverticulitis. The histological differential diagnoses of IMTs depend on the dominant basic histological patterns, which involve the extent of proliferation and sclerosing, and the extent to which the IMT is fibromyxoid/vascular3. It is important to distinguish IMTs from other lesions in the family of inflammatory pseudotumors, as well as from non-neoplastic fibrosclerosing processes and malignant neoplasms with a prominent inflammatory infiltrate, e.g. fibromatosis, sarcoma, gastrointestinal stromal tumor, and mesenteric pannicilitis6. Immunohistochemically, the spindle cells in consideration in the diagnostic process of IMT are presented in Table 1. The tumor is reactive to antibodies directed against vimentin, smooth muscle actin, and muscle specific actin in the majority of cases. Anaplastic lymphoma kinase expression is detected in approximately 50% of cases2,4,6. The frequency of this finding decreases with age.
A possible association with Wilms’ tumor has previously been suggested and different theories have been proposed. One theory is a shared genetic predisposition; another theory is that the treatment of Wilms’ tumor involving radiation and chemotherapy may damage the tissue and predispose the patient to development of IMTs. Notable that the latency time in this case is remarkable longer from the other reported cases2. The IMTs were at first considered a postinflammatory condition but is now acknowledge as a distinct neoplasm based on clonal rearrangements involving chromosome 2p6. The IMTs have been classified by WHO as a neoplasm of uncertain behavior2. The tumors are widely acknowledged as benign but with a potential to recur at the primary site. Approximately 8–25% recurs locally1,4. Rare examples are described with tumors undergoing malignant transformation2 and/or with metastasis4. Several studies have attempted to find predictors of aggressive behavior in IMTs without success6. However, it has been suggested that IMTs with a proliferating pattern, a multinodular presentation, or a manifest myofibroblastic or fibroblastic phenotype are more likely to recur4. Furthermore, IMTs arising in the gastrointestinal tract are more likely to recur compared with similar tumors arising elsewhere7. Anaplastic lymphoma kinase expression is associated with a lower risk of metastasis6,7. Complete surgical resection is the treatment of choice, and re-excision is the preferred therapy for local recurrence7. To our knowledge no guideline on IMT follow-up is available.
Adapted from 6, to consider in the diagnostic process of IMT.
An IMT in the terminal ileum can mimic Meckel’s diverticulitis and the clinical manifestations can include intestinal obstruction due to the formation of a conglomerate of small intestinal loops. Furthermore, IMTs should be considered as a diagnostic possibility in patients with a past medical history of Wilms’ tumor2.
Written informed consent for publication of clinical details and clinical images was obtained from the patient.
JR contributed to the conception of the article. JL, RSS, JR, and JB contributed to the design of the work. JL contributed to the acquisition of data and prepared the first draft of the manuscript. RSS provided expertise in pathology and acquisition of clinical photographs. JL, RSS, JR, and JB were involved in the revision of the draft manuscript and have agreed to the final content.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Gastrointestinal, hepatobiliary, pancreatic pathology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 25 Sep 17 |
read | read |
|
Version 1 15 May 17 |
||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)